Recently, Acta Materialia published the new research study about nickel-based alloy from Professor Jun Wang’s group with the title of “Unveiling the mechanism of yttrium-related microstructure inhibiting or promoting high-temperature oxidation based on Ni-Al-Y alloys”. The first author of the paper is PhD student Yun Wu. Professor Jun Wang and Assistant Researcher Maodong Kang are the co-corresponding authors. After publishing research study in Scripta Materialia, Prof. Wang’s group again published their important finding on the nickel-based superalloy in top journal in the field of metal materials. In addition, the related research results have been allowed for issuance as a patent by US Patent and Trademark Office, with a title of “Ni-Al-RE ternary eutectic alloy and preparation process therefor”, Application No. of 16/630848, and Patent No. of 10988833 (Fig. 1).
Fig. 1 US Patent of the relevant study
The development of high thrust aircraft engine and advanced ultra-supercritical coal-fired power plant is inseparable from the support of gas turbine and key components. Nickel-based superalloys featured by an excellent combination of high-temperature strength, toughness, ductility, and oxidation resistance, play a vital role in keeping these components reliable during service. However, the long-term and complex high-temperature service environment still makes nickel-based superalloys face great challenges, one of which is oxidation resistance. The introduction of reactive component (such as Y) has been proved to be effective in improving high-temperature oxidation resistance. This published study is dedicated to reveal the effect of rare earth Y on the high-temperature oxidation behavior of nickel-based alloys, including the evolution of oxide and thermodynamic calculations, the structure of oxide layer.
This study introduces the discovery of microstructure depended external and internal oxidation behaviors based on four simplified Ni-Al-Y alloys during isothermally exposing at 800 °C/1000 °C in air. Y-related microstructures are found to strongly drive the oxidation process. Original-Ni5Y compound with strong tendency to precipitate secondary γ-Ni strip is favorable to the formation of inner oxidation layer containing mixed oxide particles, whereas original-γ-Ni phase drives the formation of outer scale of NiO. Dense and well-configured lamella-like phase boundaries (PBs) are beneficial to inhibiting the development of the inner layer, compared to coarse and irregular PBs. According to Wagner’s critical Al concentration, the improved resistance to inner oxidation of minor-Y-addition alloy has been explained in detail. When the temperature increases, the diffusion coefficient of Al increases, the ability to form Al2O3 increases significantly, and the resistance to internal oxidation increases. This study provides a new sight for the design of rare earth micro-alloyed nickel-bases superalloy, which will help to further improve the oxidation resistance (Fig. 2-5).
The work was supported by National Natural Science Foundation of China (Nos. 51971142, 52031012), National Science and Technology Major Project of China (No. 2017-VI-0013-0085) and Aeronautical Science Foundation of China (No. 2018ZE57012). Importantly, the work was also supported by Oxford Instruments plc and Instrumental Analysis Center of Shanghai Jiao Tong University.
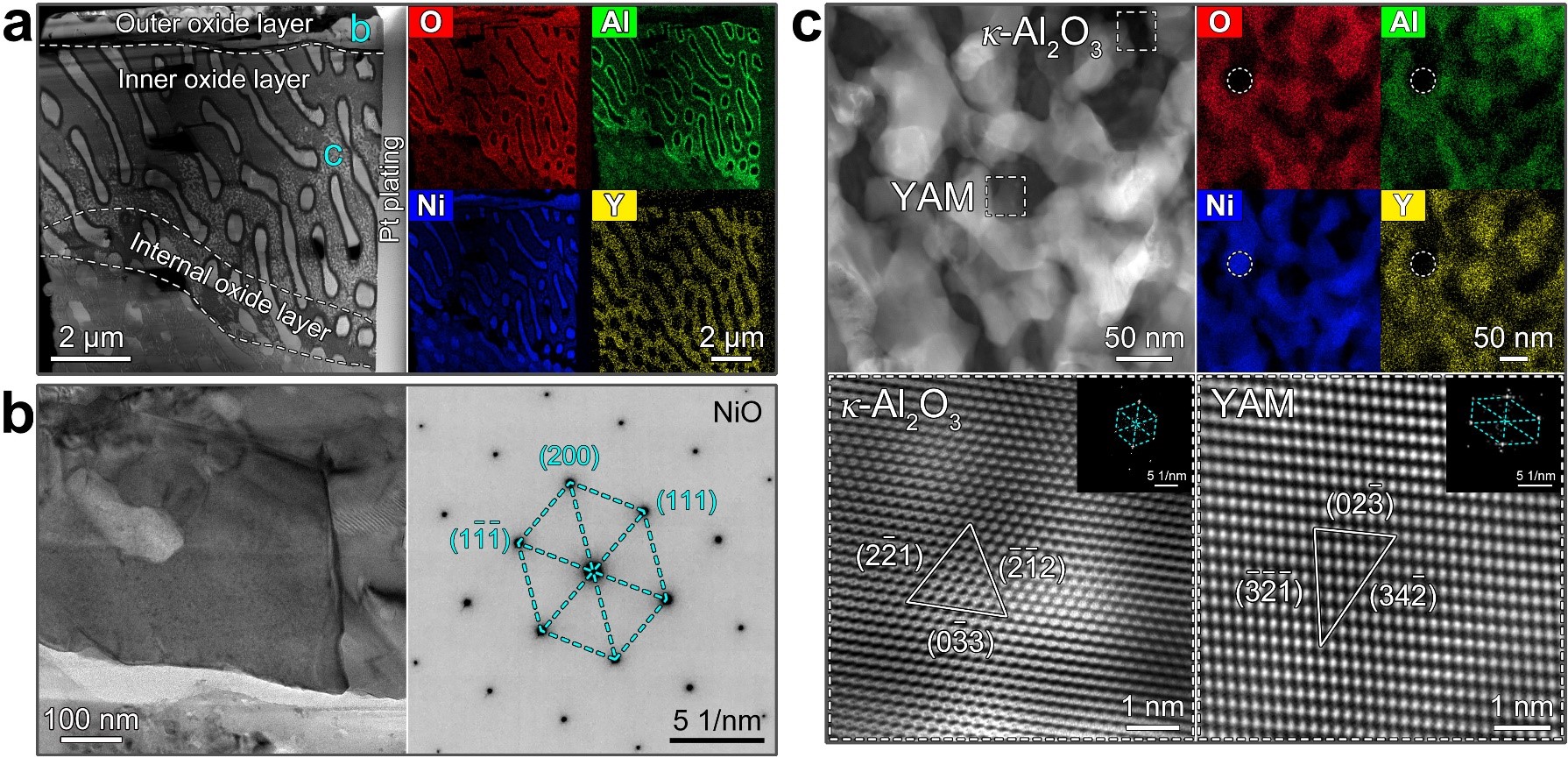
Fig. 2 Microstructures of the 1 h/800℃ oxidized 22Al10Y alloy. (a) Overall morphology and element distribution of the oxide layer. (b) NiO at the outermost surface. (c) Mixed oxides within the Ni5Y phase.
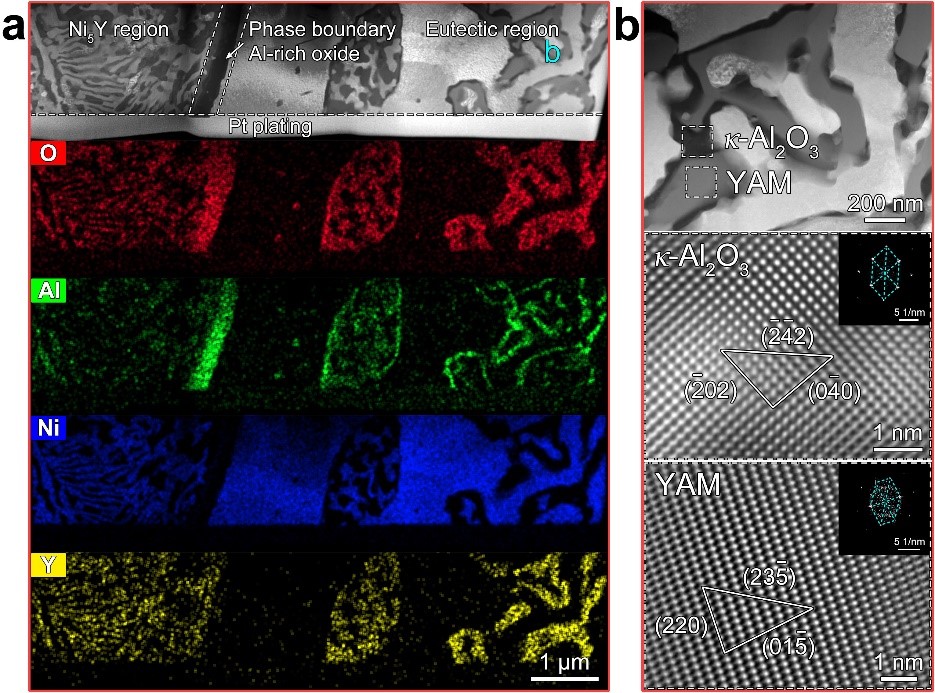
Fig. 3 Microstructures of the 200 h/800℃ oxidized 10Al10Y alloy. (a) Local morphology and element distribution of the internal oxide layer. (b) Mixed oxides in the eutectic region.
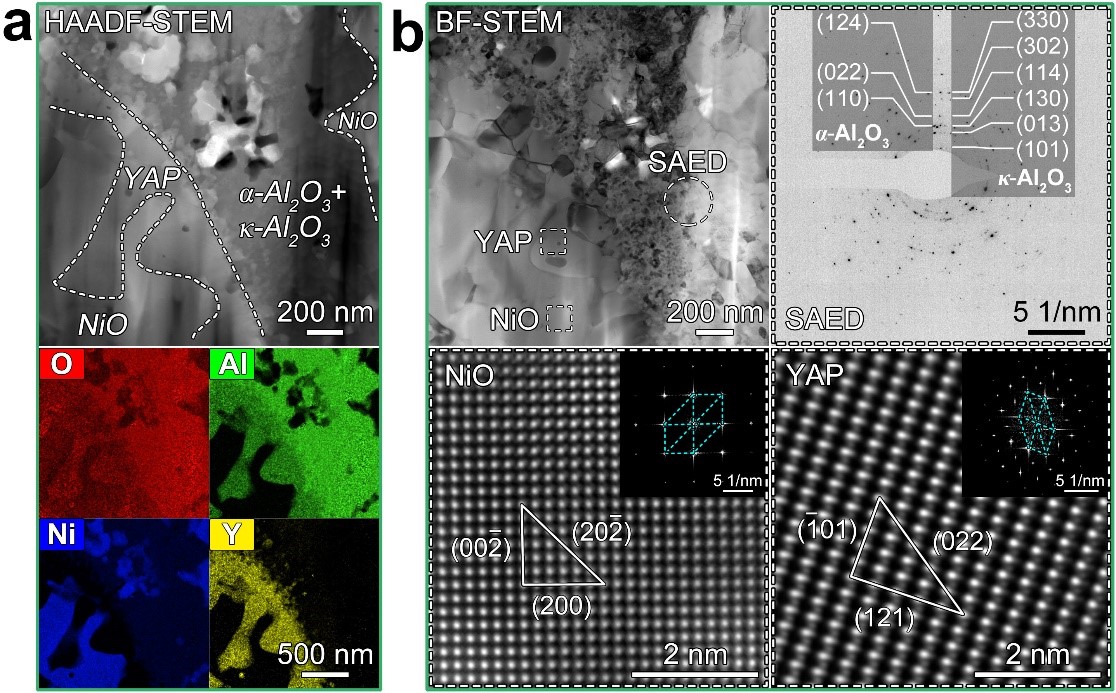
Fig. 4 Microstructures of the 200 h/1000℃ oxidized 10Al0.5Y alloy. (a) Local morphology and element distribution of the internal oxide layer. (b) Mixed oxides at the PB.
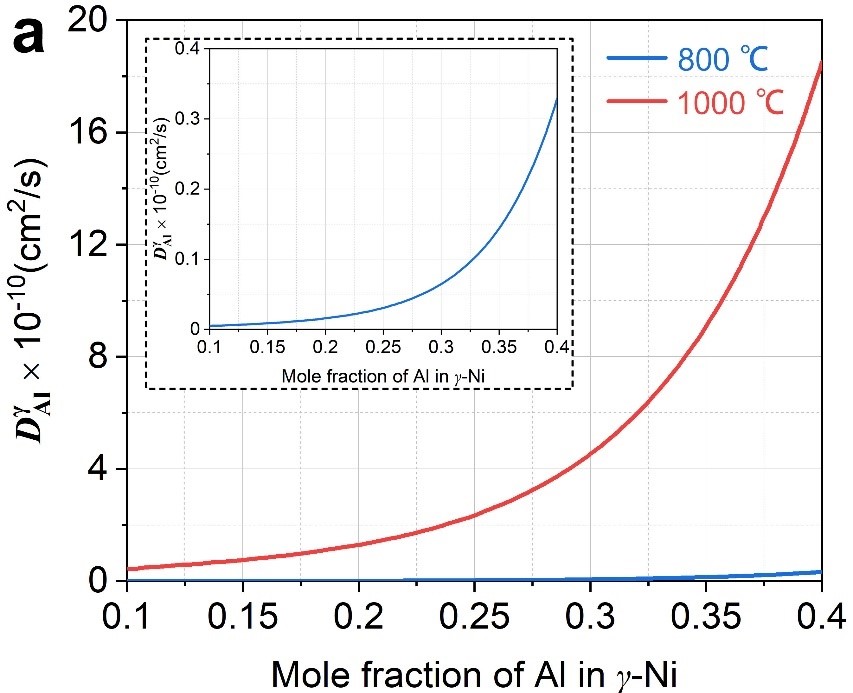
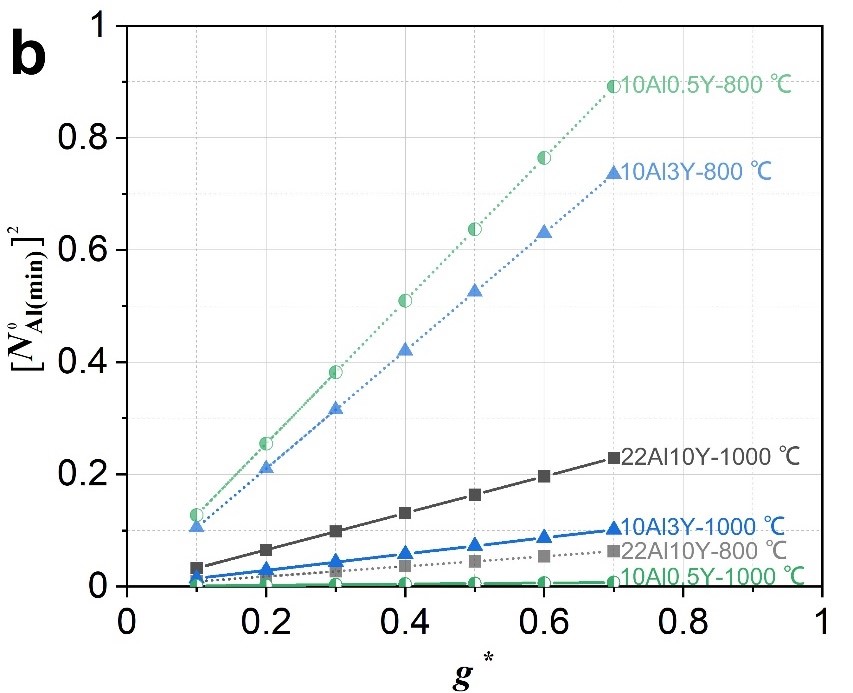
Fig. 5 (a) Diffusion coefficient of Al in γ-Ni. (b) Relationship between critical concentration of Al and g* based on Wagner’s criterion.
