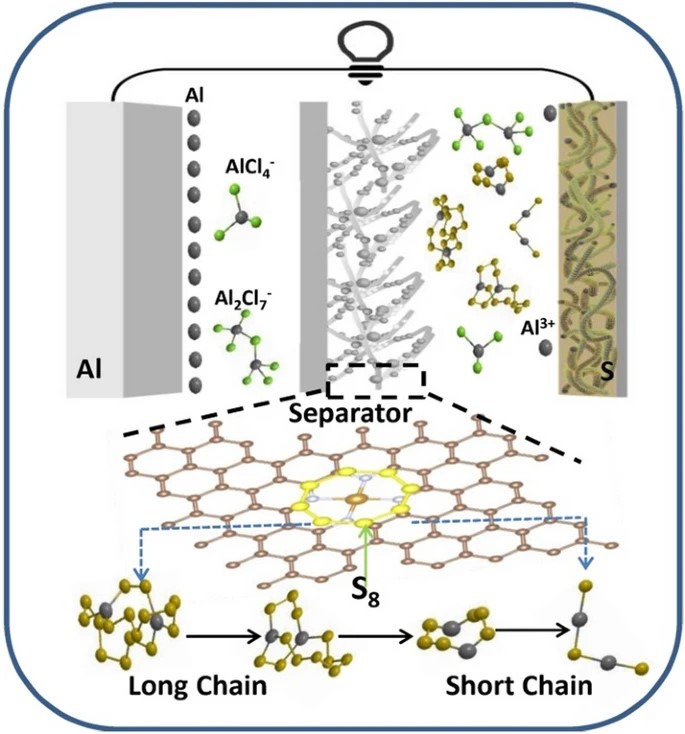
Rechargeable aluminum–sulfur (Al–S) batteries have been considered as a highly potential energy storage system owing to the high theoretical capacity, good safety, abundant natural reserves, and low cost of Al and S. However, the research progress of Al–S batteries is limited by the slow kinetics and shuttle effect of soluble polysulfides intermediates, which severely limits the practical application and commercialization of Al–S batteries.
Atomically Dispersed Iron Active Sites Promoting Reversible Redox Kinetics and Suppressing Shuttle Effect in Aluminum–Sulfur Batteries
Fei Wang, Min Jiang, Tianshuo Zhao, Pengyu Meng, Jianmin Ren, Zhaohui Yang, Jiao Zhang, Chaopeng Fu*, Baode Sun
Nano-Micro Letters (2022)14: 169
https://doi.org/ 10.1007/s40820-022-00915-4
HIGHLIGHTS
1. Fe single atoms supported on porous carbon nanofiber are prepared by spatial confinement.
2. The iron single atoms supported on porous nitrogen-doped carbon nanofibers (FeSAs-NCF) can promote the reversible conversion
between aluminum polysulfides.
Content Introduction
Recently, Chaopeng Fu's research team at Shanghai Jiao Tong University has prepared a porous nitrogen-doped carbon nanofibers (FeSAs-NCF) loaded with iron single atoms by plasma etching and electrostatic spinning and directly coated onto the separator to enhance the electrochemical performance of Al-S batteries. The HAADF-STEM and XANES characterizations clearly reveal that the active center is the atomically dispersed iron active sites (Fe–N4). The Al-S cell with the Fe-SAs-NCF shows an improved specific capacity and enhanced cycle stability, with the specific capacity remaining 320 mAh g-1 after 1500 cycles at a current density of 1000 mA g-1. As evidenced by experimental and theoretical results, the atomically dispersed iron active centers on the separator can chemically adsorb the polysulfides and accelerate reaction kinetics to inhibit the shuttle effect and promote the reversible conversion between aluminum polysulfides, thus improving the electrochemical performance of the Al–S batteries.
Graphic guide
it displays nanofiber structure and there are abundant pores inside the nanofibers (Fig. 1b-c) through the scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
HAADF-STEM images display the homogeneously distributed Fe single atoms, which are clearly identified throughout the carbon nanofibers in the enlarged image. Additionally, the elemental mappings of the FeSAs-NCF evidence the homogeneous distribution of Fe, C and N in the nanofibers. The absence of any Fe-related phase in the XRD pattern confirms the atomic dispersion of Fe in the N-doped carbon nanofibers. The XANES spectra of FeSAsNCF is close to that of FePc, which indicates that the Fe single atom is in the oxidation state. The Fourier-transformed R-space diagram shows that FeSAs-NCF has a prominent peak at 1.5 Å and there is no peak corresponding to Fe-Fe bond at 2.09 Å, indicating that the Fe single atom exists mainly as Fe-N. The test results of XANES, EXAFs and the corresponding wavelet transform of Fe indicate that the active center is an atomically dispersed Fe-N4 structure.
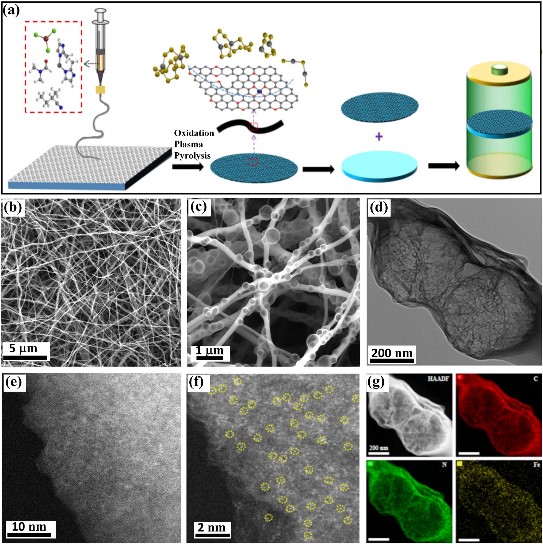
Fig. 1 a Synthetic illustration of the FeSAs-NCF. b, c SEM, d TEM, e, f aberration-corrected HAADF-STEM images and g the corresponding element mappings of the FeSAs-NCF.
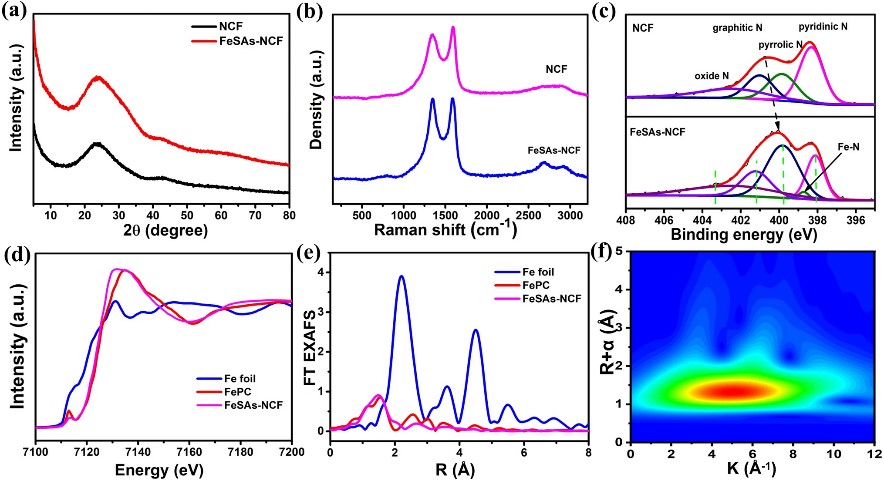
Fig. 2 a XRD patterns, b Raman spectra and c N 1s XPS of the FeSAs-NCF and NCF. d XANES and e FT EXAFS spectra of the FeSAs-NCF, Fe foil and FePc. f Wavelet transforms of the FeSAs-NCF.
Electrochemical performance of Al-S batteries
The representative cyclic voltammetry (CV) curves clearly demonstrate the reversible redox reactions between aluminum and sulfur in the Al–S batteries (Fig. 3a) [23]. Meanwhile, the Al–S battery with the FeSAs-NCF displays larger redox peak current densities and a smaller peak potential separation than the cell with NCF and the blank cell, demonstrating the FeSAs-NCF can facilitate the redox reaction between Al and S and accelerate the redox kinetics of converting sulfur into aluminum sulfides. The role of FeSAs-NCF in promoting charge transfer capability was evaluated by electrochemical impedance spectroscopy (EIS). The typical galvanostatic charge/discharge curves display that the cell with FeSAs-NCF shows a higher discharge voltage and delivers a larger discharge capacity than the cell with NCF and the blank cell, indicating the lowest energy barrier of the conversion reaction between charge and discharge products. the cell with FeSAs-NCF delivers an initial capacity of 1130 mAh g−1 at 100 mA g−1 and remains a stable capacity of 780 mAh g−1 after 100 cycles. However, the battery with NCF and blank cell only remain the specific capacities of 280 and 70 mAh g−1 after 100 cycles, respectively. Furthermore, the battery with FeSAs-NCF demonstrates good cycling stability and the specific capacity reaches 320 mAh g−1 at a high current density of 1000 mA g−1 after 500 cycles.
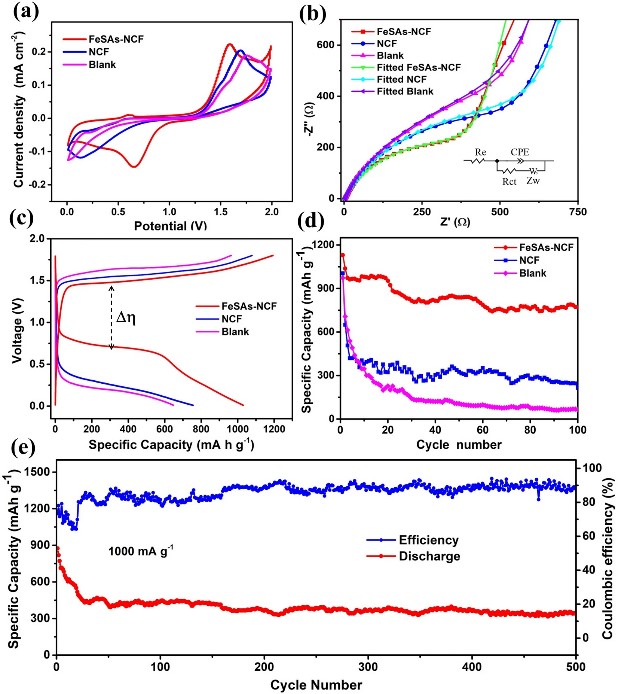
Fig. 3 a CV curves, b Nyquist plots, c charge–discharge curves and d cycling stability of the Al–S batteries with FeSAs-NCF and NCF, and the blank cell, e cycling stability of the cell with FeSAs-NCF at 1000 mA g-1.
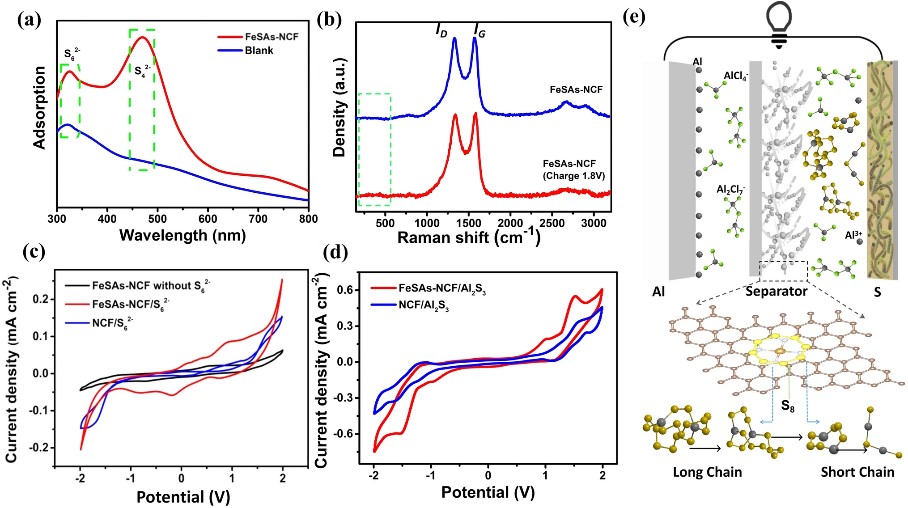
Fig. 4 a UV–Vis spectra of the electrolytes in the Al–S batteries with FeSAs-NCF and the blank cell after charge/discharge. b Raman spectra of pristine FeSAs-NCF and the FeSAs-NCF collected at a charge state of 1.8 V. c CV curves of the symmetric cells in the electrolyte containing S2−6. d CV curves of the symmetric cells with Al2S3. e Schematic illustration of inhibiting shuttle effect and catalyzing aluminum polysulfides of the Al–S battery with FeSAs-NCF
Moreover, compared with the NCF/Al2S3 symmetric cell, the FeSAs-NCF/Al2S3 symmetric cell shows the smallest voltage hysteresis between the oxidation and reduction peaks, confirming the atomically dispersed Fe active centers are more favorable to promote the kinetic conversion of Al2S3. All the above results experimentally verify that the FeSAs-NCF can not only suppress the shuttle effect but also catalyze the redox reactions of aluminum polysulfides for the Al–S battery. Based on the above analysis and discussion, the mechanism of the FeSAs-NCF inhibiting shuttle effect and catalyzing aluminum polysulfides is illustrated, and the discharge reactions on cathode side is expressed as follows:
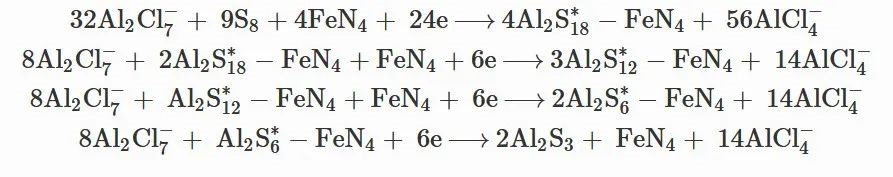
The overall reaction on
cathode side:
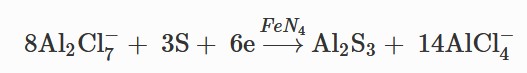
Meanwhile, the reaction on
anode side can be written below:
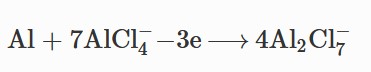
Accordingly, the overall
reaction is given below:
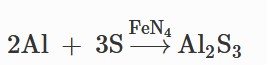
Theoretical calculation:
The first-principle calculations were performed to further reveal the improved reaction kinetics of the aluminum polysulfides. The Gibbs free energies were calculated of each reaction on both the NCF and FeSAs-NCF substrates, and the corresponding Gibbs free energy profiles are shown in Fig. 5b. After the spontaneous exothermic process of converting S8 to Al2S18, where the steps of the formation of Al2S12, Al2S6 and Al2S3 are either endothermic or nearly thermoneutral, the largest positive Gibbs free energy of the formation of Al2S3 from Al2S6 reveals that this process is the rate-determining step in the entire discharge reaction. The Gibbs free energy on the FeSAs-NCF (2.65 eV) is lower than that of on the NCF (3.20 eV) for the reduction of Al2S6, demonstrating the reduction of S is thermodynamically more favorable on the FeSAs-NCF than on the NCF substrate.
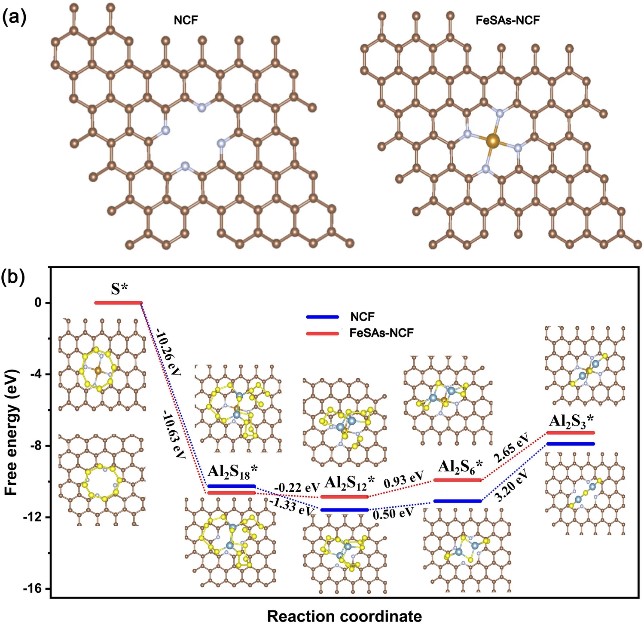
Fig. 5 a The optimized structure models of NCF and FeSAs-NCF and b Energy profiles for the reduction of sulfur to aluminum sulfides on NCF and FeSAs-NCF
In summary, iron single atoms dispersed in nitrogen-doped carbon nanofibers have been successfully prepared and used as a unique interlayer to modify the separator for Al–S batteries. The HAADF-STEM and XANES characterizations confirm the iron is atomically dispersed with the coordination of Fe–N4. The Al–S battery with the FeSAs-NCF demonstrates significantly enhanced electrochemical performance, and the Al–S battery with FeSAs-NCF delivers a specific capacity of 780 mAh g−1. The enhanced performance is ascribed to the unique spatial configuration and chemical properties of FeSAs-NCF. Specifically, the FeSAs-NCF is functioned as a chemical and physical barrier to hinder the shuttle effect of soluble aluminum polysulfides. More importantly, the Fe single atoms can catalyze the conversion of aluminum polysulfides to promote the kinetics of the charge/discharge processes.
Author Introduction

Chaopeng Fu
Corresponding author of this article
Special Researcher of Shanghai Jiao Tong University
Main Research Fields
Metal energy materials: aluminum air battery, aluminum ion battery, aluminum carbon dioxide battery, etc.; high purity ultra-fine alumina materials; surface treatment and corrosion and protection of aluminum alloy.
Major Research Results
He worked as a postdoctoral researcher in Oxford University, UK from 2013 to 2016, and worked as a postdoctoral fellow in Nanyang Technological University, Singapore in 2012. He received his Ph.D. and B.S. degrees from Hunan University in 2011 and 2006, respectively. His research direction has been funded by the Ministry of Organization and the National Natural Science Foundation of China. His research interests include material processing and development and application of electrochemical energy storage materials and devices. He has carried out fruitful work on aluminum air power battery and its full-cycle research, development of aluminum-based energy storage devices and functional materials, and surface treatment and corrosion and protection of aluminum alloy. At present, he has published more than 70 academic papers in international famous academic journals and made many oral presentations in international academic conferences.
