Recently, the School of Materials Science and Engineering at Shanghai Jiao Tong University (SMSE, SJTU) has made new progress in the field of inorganic ductile non-metallic materials. The related findings are published online in Advanced Functional Materials with the title being “Room temperature wide-gap inorganic materials with excellent plasticity”. Professor Xun Shi and associate professor Tian-Ran Wei are the corresponding authors; The PhD student, Haoran Huang, is the first author. The collaborating institution includes the Shanghai Institute of Ceramics, Chinese Academy of Sciences.
Inorganic non-metallic materials, including semiconductors, ceramics, and glass, generally exhibit brittleness at room temperature, which greatly limits their deformability and processing capabilities. In recent years, layered crystals such as Ag2S-based materials and InSe-like layered crystals have been found to have extraordinary room-temperature plasticity. These findings have greatly changed people’s traditional understanding of the mechanical properties of inorganic materials and expanded their application scenarios in complex hetero-shaped structures. However, almost all the existing ductile inorganic materials are narrow-gap semiconductors (bandgap < 2 eV). There are few reports on plastic materials for a large number of wide-gap inorganic materials which are widely used in high-power devices, dielectric and other fields.
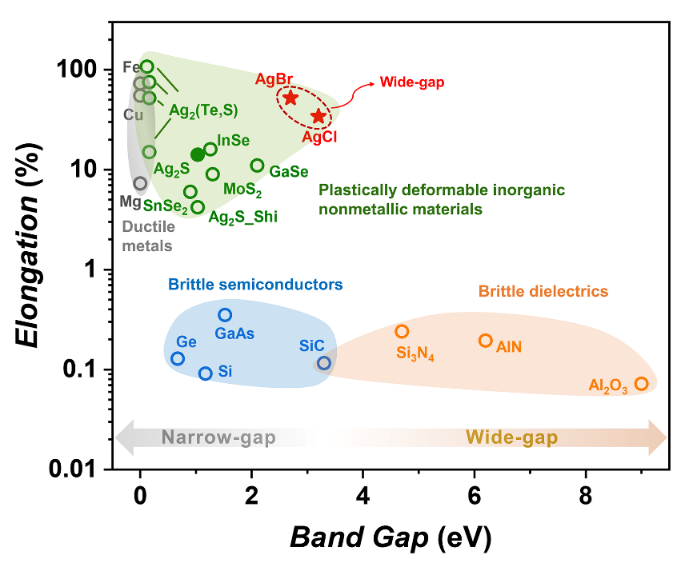
Figure 1 Elongation and band gap of different inorganic nonmetallic materials at room temperature
Inspired by the plasticity of Ag2S and its unique Ag-S bonds, the team explores plastically deformable inorganic materials based on silver chalcogenides (Ag2Q) and silver halides (AgX). Historical studies have mentioned that AgCl and AgBr have good plastic deformation capabilities, but their mechanical properties in various loading modes are unclear, and the plastic deformation mechanisms at atomic and chemical bond scales are not fully understood. This work studied the mechanical properties of six silver based polycrystalline bulk materials, AgX (X=Cl, Br, I) and Ag2Q (Q=S, Se, Te). It was found that the wide-gap materials, AgCl and AgBr, exhibit excellent plasticity under various load conditions (Figure 1 and Figure 2): room temperature tensile elongation reaching 30-50%, compression strain greater than~60%, bending strain exceeding 25%, rolling extension up to 4200%, and rolling reduction rate larger than 97%. In contrast, the majority of inorganic non-metallic materials are usually brittle at room temperature, with a tensile elongation of less than 1%.
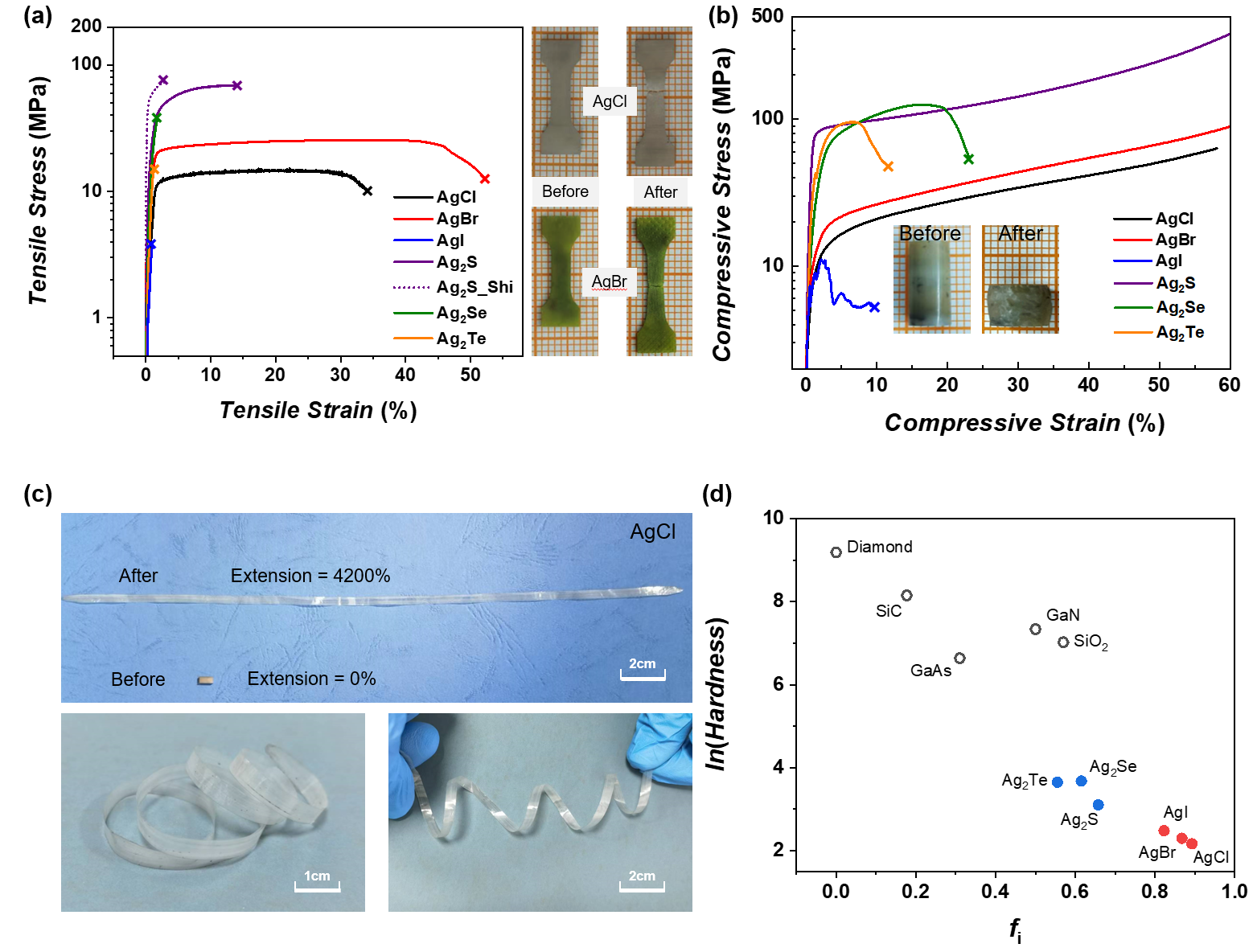
Figure 2 Mechanical properties of Ag2Q and AgX at room temperature. (a) Tensile stress-strain curves, as well as photos of AgCl and AgBr before and after tension; (b) Compression stress-strain curve with the photos of AgCl before and after compression; (c) Photos of AgCl before and after rolling; (d) Relationship between the natural logarithm of hardness and ionicity.
Theoretical calculations have demonstrated that both AgCl and AgBr have multiple slip systems with larger surface energy (cleavage energy) and smaller generalized stacking fault energy (slip energy barrier). In addition, their crystal structures exhibit high symmetry, providing more slip systems that can be activated. Charge analysis shows that when the atomic layer slips relatively to each other, the electron cloud spatial distribution of Cl - and Br - ions undergoes a certain polarization, which suppresses the repulsive effect of the like charges and keeps the chemical bonding at a high level. However, the polarization effect of the brittle material NaCl with the same structure is very weak. From a more fundamental perspective, there is a general relationship among various inorganic materials, including AgX and Ag2Q materials, whereby stronger ionicity usually leads to lower hardness and enhanced plasticity (Figure 2d). This is attributed to chemical bonds, including ionic bonds based on Coulomb forces that are diffusive and non-saturated, distinct from covalent bonds. This is helpful to the continuous disruption and re-construction of the bonds during slip. Therefore, the relatively strong ionicity (non-covalency) combined with a certain degree of polarization of anions may be the reason for the excellent plasticity of AgCl and AgBr. This work will deepen the scientific understanding of inorganic ductile functional materials, which is helpful for exploring more wide-gap plastically deformable inorganic non-metallic materials, transforming the processing and manufacturing methods of inorganic materials, and expanding application scenarios.
This study was supported by the National Natural Science Foundation of China (T2122013, 52232010) and the Basic Research Project of the Shanghai Science and Technology Committee (20JC1415100).
Reference
Haoran Huang, Heyang Chen, Zhiqiang Gao, Yupeng Ma, Kunpeng Zhao, Tian-Ran Wei,* Xun Shi.* Room-temperature wide-gap inorganic materials with excellent plasticity. Advanced Functional Materials, 2023, doi: 10.1002/adfm.202306042
