Background
Magnesium (Mg) alloys are widely utilized in aerospace, transportation, biomedical, and electronics industries due to their lightweight properties. However, their limited corrosion resistance has hindered extensive industrial applications. Existing research on corrosion-resistant Mg alloys largely remains in the experimental observation stage, with an insufficient understanding of the corrosion mechanisms at the atomic level. Furthermore, the development of novel corrosion-resistant Mg alloy systems primarily relies on empirical rules, lacking adequate theoretical guidance and basis. With the rapid advancement of information technology in the twenty-first century, computational materials science has emerged as the mainstream trend in new material development. Meanwhile, the development of high-throughput computation and data science has significantly accelerated the pace of new material research. Despite this, due to the intricate nature of Mg corrosion, systematic studies on Mg corrosion, particularly regarding the effect of intermetallic phases on cathodic corrosion behavior, are still insufficient.
Introduction
Prof. Hong Zhu and Prof. Xiaoqin Zeng, from Shanghai Jiao Tong University, employed first-principles calculations to investigate the influence of different binary Mg intermetallics on cathodic corrosion reactions. In this study, the authors applied active learning techniques to accelerate the screening of intermetallics that can inhibit the cathodic hydrogen evolution reaction (HER) in Mg alloy corrosion. The adsorption energy of hydrogen atoms on the intermetallic surfaces was utilized as a descriptor for the HER rate. By utilizing the geometric and chemical characteristics of hydrogen atoms' Voronoi neighbors on unoptimized adsorption structures, machine learning models predicted the hydrogen adsorption energies for various adsorption configurations. Remarkably, the prediction error of the H adsorption energy for the strong/weak adsorption configuration is 0.196 eV (MAE) with the training set size less than 1% unknown data set. Furthermore, the authors discovered that surfaces with strong hydrogen adsorption transferred more electrons to hydrogen atoms than surfaces with weak adsorption. Lastly, based on the surface stability of intermetallics and the predicted hydrogen adsorption energies, the authors ranked the capability of all binary intermetallic compounds in Mg alloys to inhibit the HER. The relevant article, entitled "Accelerated discovery of magnesium intermetallic compounds with sluggish corrosion cathodic reactions through active learning and DFT calculations," was published in Acta Materialia.
Paper link
https://doi.org/10.1016/j.actamat.2023.119063

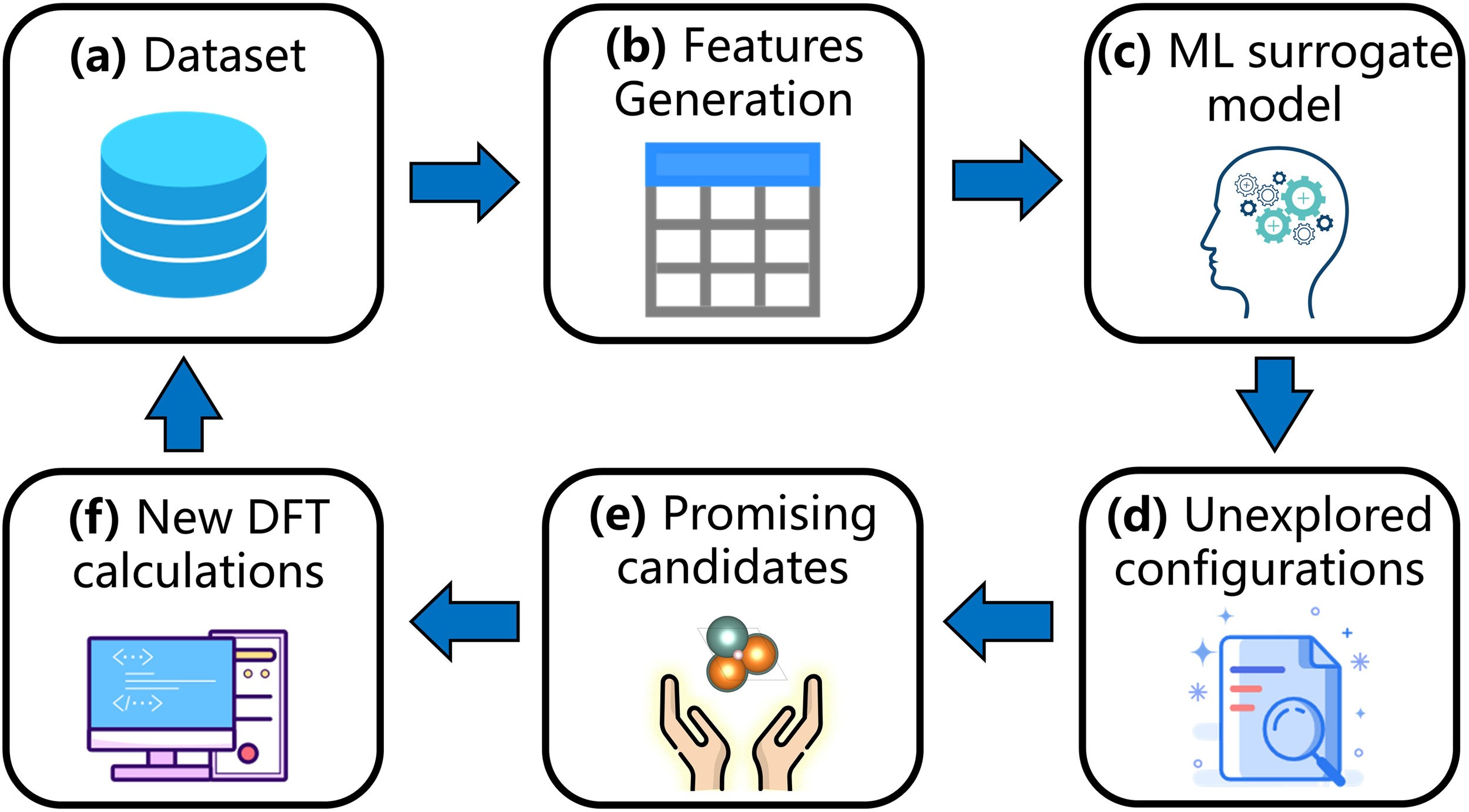
Fig. 1. A schematic diagram of active learning design framework to accelerate the discovery of corrosion-resistant binary Mg alloys. (a) The original dataset was collected from our previously developed dataset. (b) Adsorption features, including geometric and chemical features related to ![]() , were extracted from the initial adsorption configuration based on Voronoi polyhedron. (c) ML surrogate models were trained based on input features. (d) Adsorption configurations of unexplored Mg binary intermetallics were generated by pymatgen. (e) ML models were applied to predict
, were extracted from the initial adsorption configuration based on Voronoi polyhedron. (c) ML surrogate models were trained based on input features. (d) Adsorption configurations of unexplored Mg binary intermetallics were generated by pymatgen. (e) ML models were applied to predict ![]() and promising candidates with high or low
and promising candidates with high or low ![]() were picked. (f) According to prediction, new DFT calculations were performed and the obtained
were picked. (f) According to prediction, new DFT calculations were performed and the obtained ![]() were added to the dataset.
were added to the dataset.
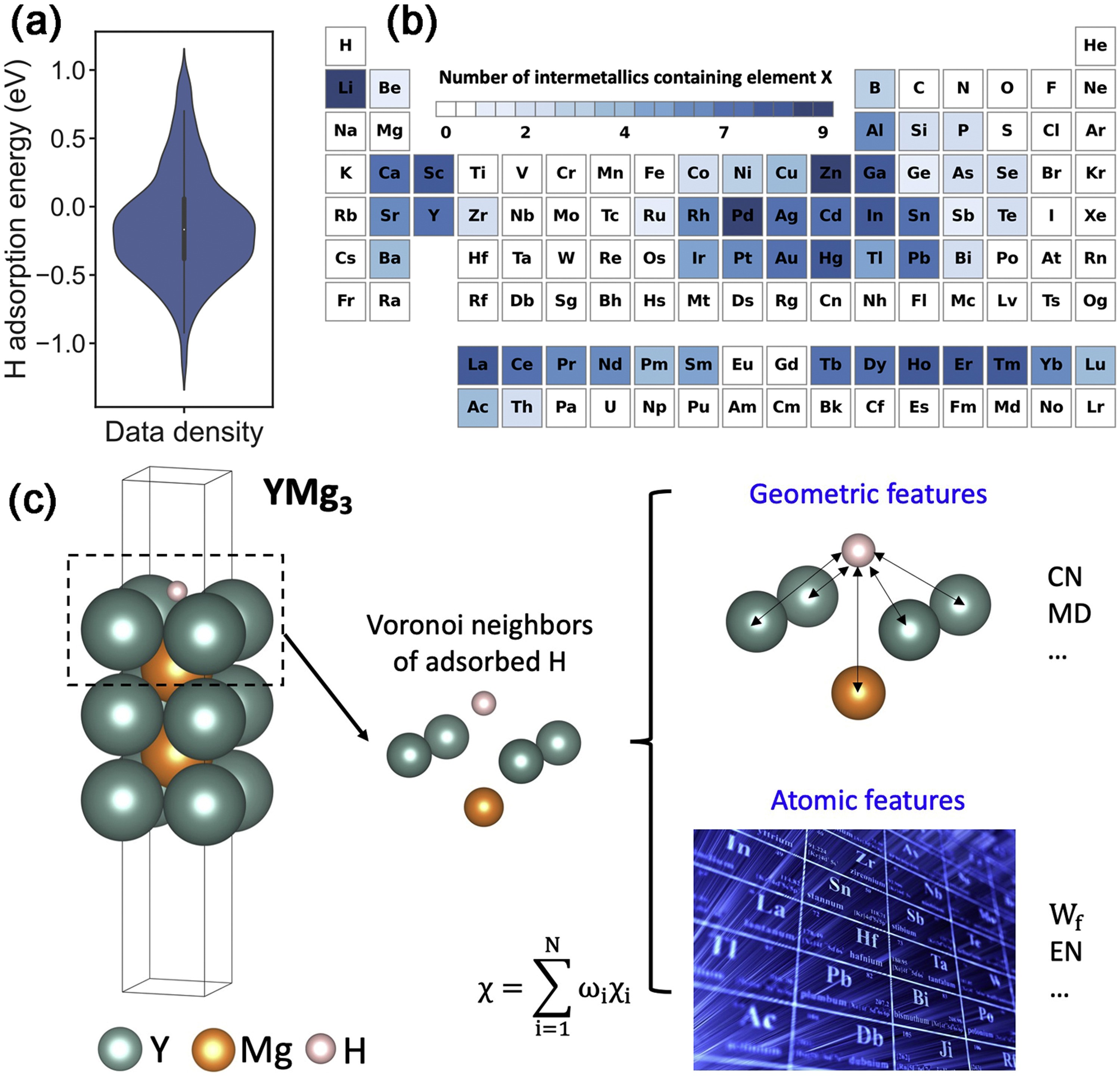
Fig. 2. (a) The statistical distribution of the DFT calculated ![]() from the initial dataset. (b) The number of intermetallics containing element X. The white boxes indicate that no stable or semi-stable intermetallics are found in Material Project database. The darker color of an alloy system represents a greater number of stable or semi-stable intermetallics than other alloy systems. (c) Method to extract the geometric features and chemical features from H adsorption configuration. Take YMg3 as an example, the local adsorption environment, namely H adatom and its surrounding Voronoi neighbors, is firstly extracted by Voronoi analysis. Then, geometric features of the local adsorption environment are calculated based on the geometry of Voronoi polyhedron. Chemical features of the local adsorption environment are calculated by the weighted sum of the elemental feature of each Voronoi neighbor. The weights are obtained from the solid angle of the Voronoi polyhedron.
from the initial dataset. (b) The number of intermetallics containing element X. The white boxes indicate that no stable or semi-stable intermetallics are found in Material Project database. The darker color of an alloy system represents a greater number of stable or semi-stable intermetallics than other alloy systems. (c) Method to extract the geometric features and chemical features from H adsorption configuration. Take YMg3 as an example, the local adsorption environment, namely H adatom and its surrounding Voronoi neighbors, is firstly extracted by Voronoi analysis. Then, geometric features of the local adsorption environment are calculated based on the geometry of Voronoi polyhedron. Chemical features of the local adsorption environment are calculated by the weighted sum of the elemental feature of each Voronoi neighbor. The weights are obtained from the solid angle of the Voronoi polyhedron.

Fig. 3. (a) The average performance of ML models with 25 input features. The dataset is randomly divided into 80% training set and 20% test set. (b) The average MAE of 40 new predicted (including 20 strong adsorption cases and 20 weak adsorption cases for each iteration) for 5 active learning iterations.
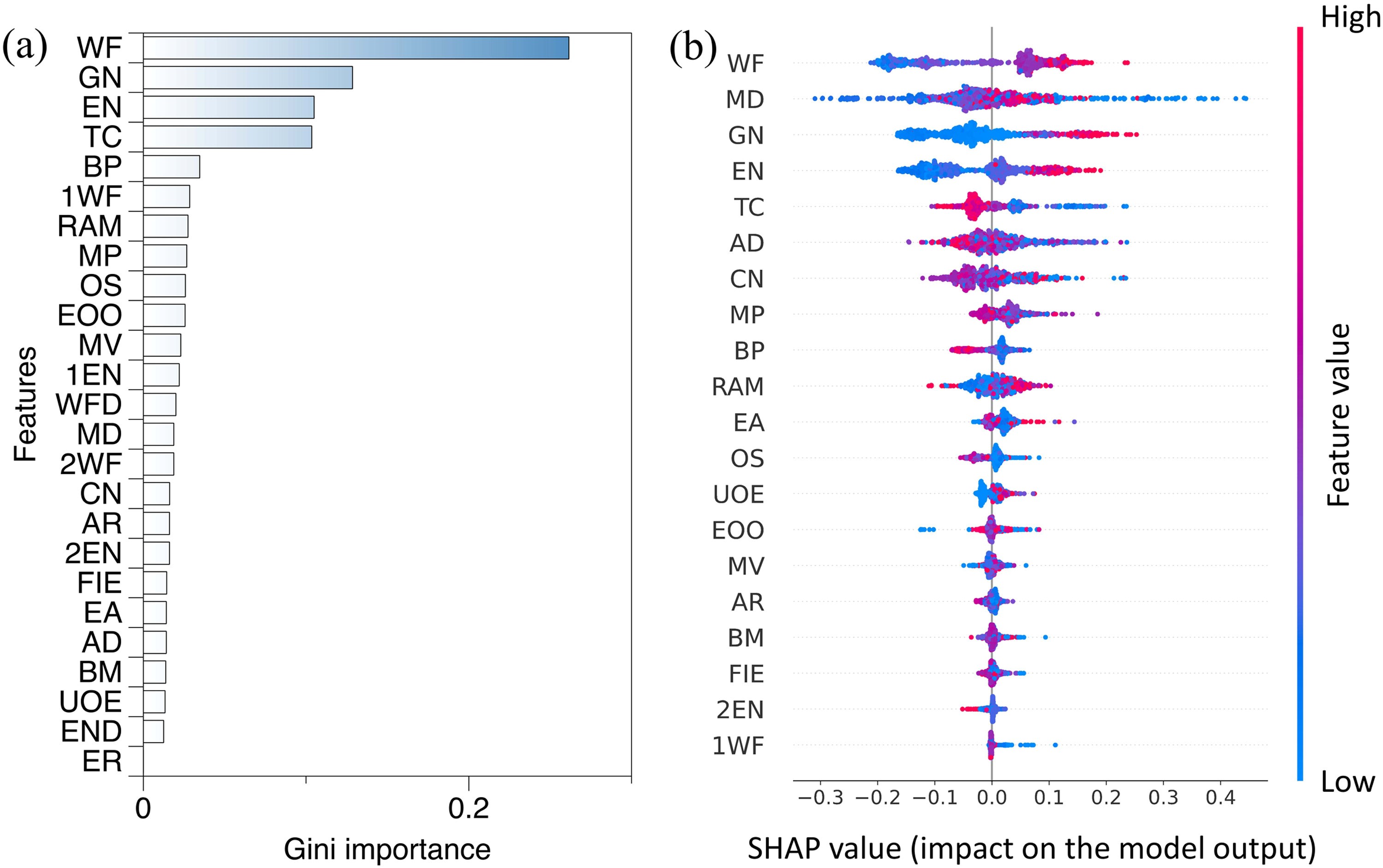
Fig. 4. (a) The feature importance obtained from XGBoost based on Gini importance. (b) Feature importance obtained by SHAP (Shapley Additive Explanations).
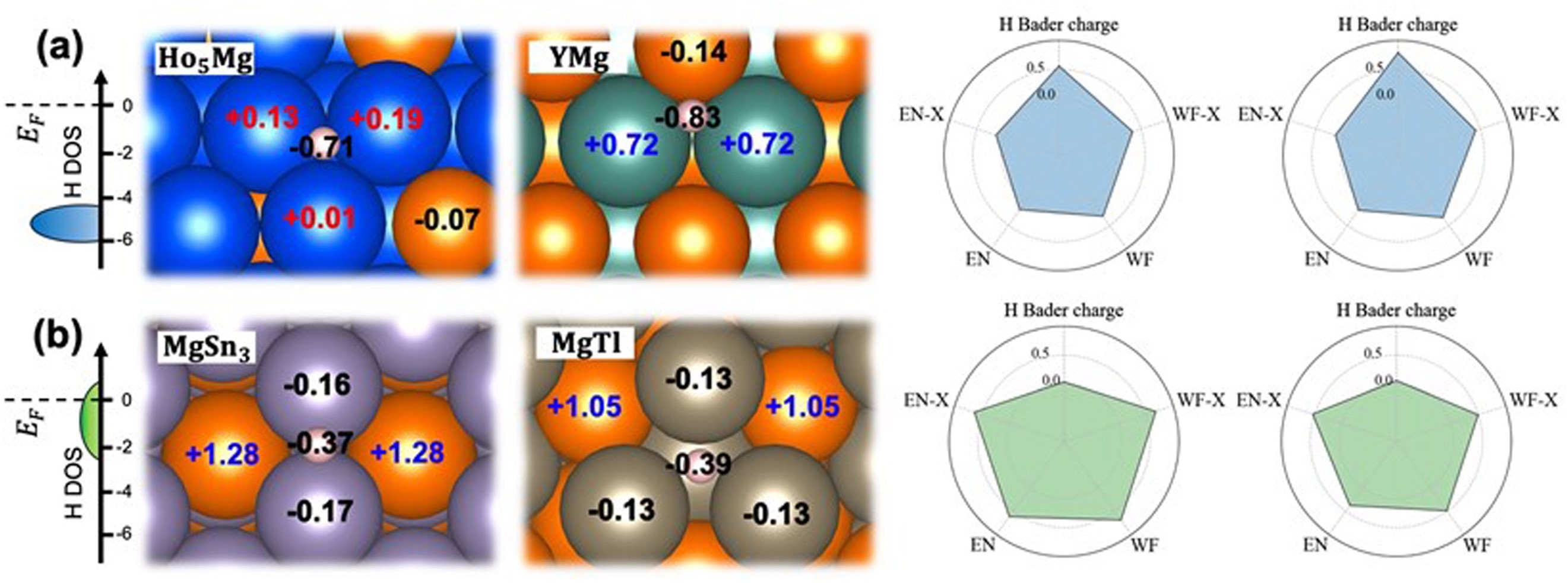
Fig. 5. Bader charge analysis and surface properties of (a) strong and (b) weak H adsorption surfaces. The left subfigs. are the schematic draw of density states of adsorbed H. The EN-X and WF-X on the right panel are the electronegativity and work function of the second element in binary Mg intermetallics. EN and WF are the weighted average electronegativity and work function of H adatom Voronoi neighbors. All the properties are normalized between 0 and 1.
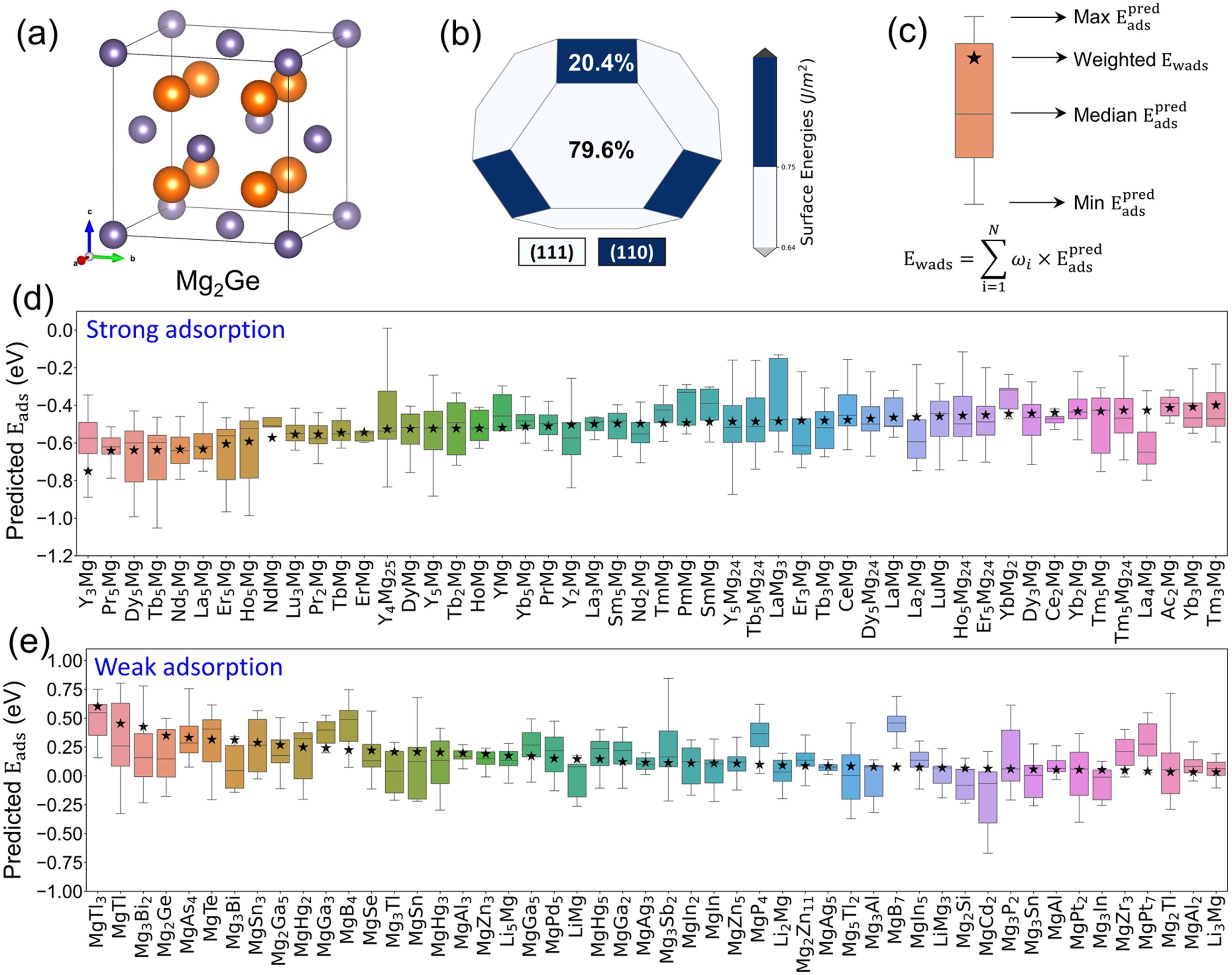
Fig. 6. The screened binary Mg intermetallics that can hinder the cathodic HER, Mg2Ge is used as an example to illustrate how to measure the ability of an intermetallic compound to inhibit the cathodic corrosion reaction of Mg alloys. (a) The unit cell of Mg2Ge where the orange atom is Mg and purple atom is Ge. (b) The constructed Wulff shape of Mg2Ge crystal. The surface energy is in the unit of J/m2. The percentage represents the proportion of each crystal plane. (c) XGBoost predicted adsorption energy range of an intermetallic with different surfaces and different adsorption sites. The intermetallics are ranked by weighted adsorption energy (), shown as black stars, which is calculated by the weighted average of the most negative ML predicted on each surface and the corresponding surface ratio. (d) Top 50 intermetallics with strong H adsorption. (e) Top 50 intermetallics with weak H adsorption.
Conclusion
The present work was conducted to discover the corrosion-resistant binary Mg alloys with intermetallic compounds showing sluggish HER by means of density functional theory computation and active learning. Active learning has been shown to significantly reduce the number of training samples while maintaining the prediction accuracy (MAE of 0.196 eV) of the H adsorption energy with easily captured features. In order to understand the reasons for the different adsorption capacities of hydrogen atoms on the different surfaces, several representative surfaces are picked to perform Bader charge and density of states (DOS) analysis. These results indicate that the electrons transferred to the H atoms on the strong adsorption surface are greater than those on the weak adsorption surface. Finally, the ability to impede HER of intermetallics is ranked based on surface stability and predicted H adsorption energy, which provides insights for further experiments. Mg intermetallics like Y3Mg and MgTl3 and Mg2Ge etc. are considered promising candidates to decrease the corrosion cathodic reaction rate. This study not only realizes the exploration of large-scale unknown space with a small amount of training data through the idea of active learning, but also provides promising intermetallics which can inhibit the galvanic corrosion of Mg alloys from the perspective of cathodic reaction, which could drive for corrosion-resistant Mg alloy design.
Author
朱虹 (hong.zhu@sjtu.edu.cn) 副教授,上海交通大学材料基因组联合研究中心成员;材料科学与工程学院,上海交通大学密西根联合学院双聘。
Google Scholar: https://scholar.google.com/citations?user=x1BGIfEAAAAJ
课题组主页: https://sites.ji.sjtu.edu.cn/hong-zhu/
Other related paper
Wang, Y.; Xie, T.; Tang, Q.; Wang, M.; Ying, T.; Zhu, H.; Zeng, X. High-Throughput Calculations Combining Machine Learning to Investigate the Corrosion Properties of Binary Mg Alloys. Journal of Magnesium and Alloys 2022.
Xie, T.; Zhao, P.; Chen, Y.; Zhang, M.; Wang, Y.; Ying, T.; Zhu, H.; Zeng, X. Investigation on the Corrosion Behavior of Single-Phase and Binary-Phase Mg-Sc Alloys: An Experimental and First-Principles Study. Materials Characterization 2021, 179, 111294.
Wang, Y.; Xie, T.; Luo, Z.; Zhu, H.; Zeng, X. First-Principles Study of Water Decomposition and Hydrogen Evolution on MgZn2 Laves Phase. Computational Materials Science 2021, 196, 110532.
Luo, Z.; Xu, J.; Wang, Y.; Xie, T.; Zhu, H.; Ying, T.; Li, D.; Wang, L.; Zeng, X.; Wolverton, C.; et al. Theoretical Analysis of the Galvanic Corrosion Behavior of Mg-Ge Binary Alloy. Journal of The Electrochemical Society 2019, 166 (13), C421–C427.
Luo, Z.; Zhu, H.; Ying, T.; Li, D.; Zeng, X., First principles calculations on the influence of solute elements and chlorine adsorption on the anodic corrosion behavior of Mg (0001) surface. Surface Science 2018, 672-673, 68-74.
