Background
Electrochemical water splitting plays a key role in storing intermittent renewable energies (such as solar and wind energies) in the chemical energy of hydrogen and oxygen1. The oxygen evolution reaction (OER, 4OH− →O2 + 2H2O+4e− in base) at the anode is the bottleneck reaction due to the sluggish kinetics2. Identifying efficient, stable, abundant, and cost-effective catalysts for OER is challenging. Iron is an extraordinary promoter to impose nickel/cobalt (hydr)oxides as the most active oxygen evolution reaction catalysts, whereas the synergistic effect is actively debated
Introduction
Prof. Fang Song, from Shanghai Jiao Tong University, unraveled a strong link between the anodically deposited FeOxHy with the active oxygen species of the metal oxyhydroxides by investigating the substrate-dependent promoting effect of FeOxHy. Their survey on the electrochemical behavior of nine supporting metal oxyhydroxides (M(O)OH) uncovers that FeOxHy synergistically promotes substrates that can produce active oxygen species exclusively. Tafel slopes correlate with the presence and kind of oxygen species. Moreover, the oxygen evolution reaction onset potentials of FeOxHy@M(O)OH coincide with the emerging potentials of active oxygen species, whereas large potential gaps are present for intact M(O)OH. Chemical probe experiments suggest that active oxygen species could act as proton acceptors and/or mediators for proton transfer and/or diffusion in cooperative catalysis. This discovery offers a new insight to understand the synergistic catalysis of Fe-based oxygen evolution reaction electrocatalysts. The relevant article, entitled " Active oxygen species mediate the iron-promoting electrocatalysis of oxygen evolution reaction on metal oxyhydroxides," was published in Nature Communications.

Paper link
https://doi.org/10.1038/s41467-023-42646-z
Research Content
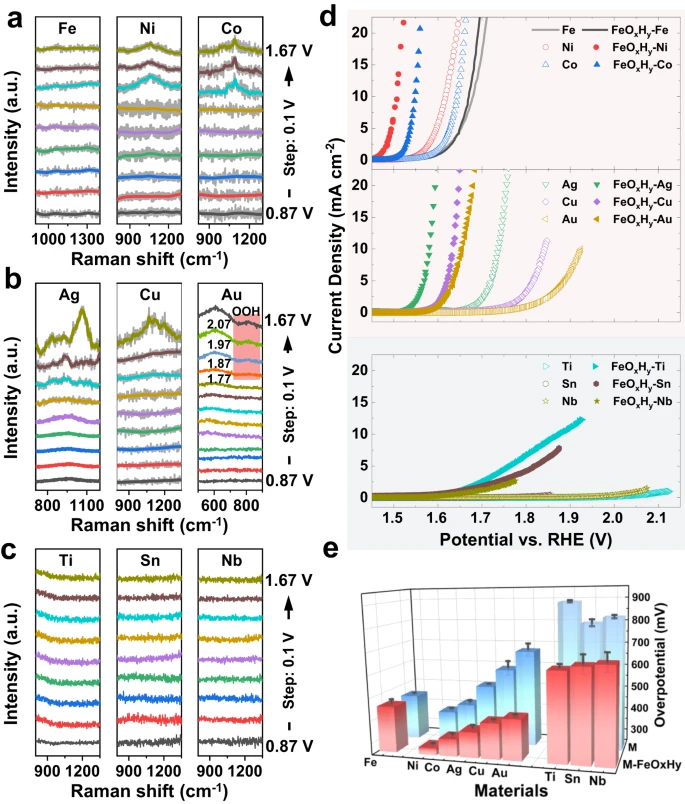
Figure 1. In situ Raman analysis and electrocatalytic performances of metal foils (M, M=Fe, Ni, Co, Ag, Cu, Au, Ti, Sn, and Sb) and FeOxHy decorated ones (FeOxHy-M). (a-c) In situ Raman spectra of (a) Fe, Ni, and Co, (b) Ag, Cu, and Au (the Raman peak in red block represents peroxyl groups (–OOH)), and (c) Ti, Sn, and Nb foils in the wavenumber region 800-1400 cm−1 (500-900 cm–1 for Au) under applied potentials ranging from 0.87 to 1.67 V vs. RHE (from bottom to top, with the interval of 0.1 V). The y-axis has arbitrary units (a.u.); (d) Polarization curves of M and FeOxHy -M. For clarity, they are divided into three subfigures according to the relative activities. Scan rate: 1 mV s–1; (e) Overpotentials required to achieve the current density of 10 mA cm–2 (for Ti, Sn, and Nb, the overpotentials for 1 mA cm–2 are summarized).
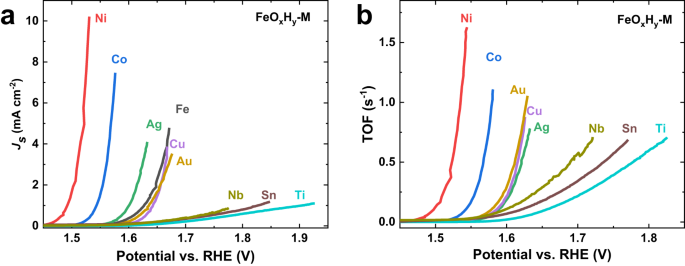
Figure 2. Specific activities and TOFs of M and FeOxHy-M. (a) Specific activities normalized by electrochemical surface areas; (b) Turnover frequencies (TOFs) assuming all the Fe atoms are the active sites. TOF of Fe foil is not available because the amount of surface Fe cannot be discriminated from the bulk substrate.
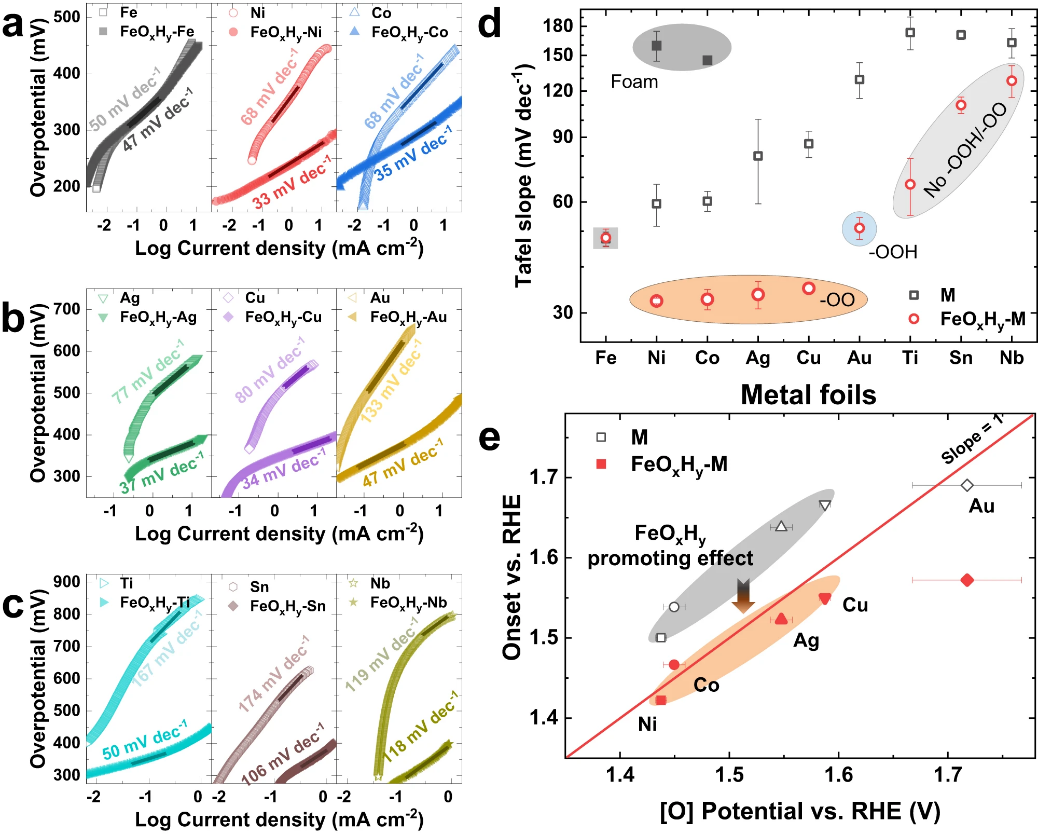
Figure 3. Correlation of catalytic performances with active oxygen species. (a-c) Tafel plottings of (a) Fe, Ni, and Co, (b) Ag, Cu, and Au, and (c) Ti, Sn, and Nb foils before and after FeOxHy decoration; (d) Tafel slopes dependent on active oxygen species. The metal foils with superoxide groups (–OO−) and peroxyl groups (–OOH) are highlighted with orange and blue backgrounds, respectively. The metal foils without any active oxygen species are highlighted with a light gray background. The Tafel slopes of Ni and Co at low overpotential regions were probed from the corresponding metal foams and are highlighted with a dark grey background; (e) OER onset potentials plotting against the emerging potentials of active oxygen species. M(O)OH and FeOxHy @M(O)OH are highlighted with gray and orange backgrounds, respectively. Ti, Sn, and Nb were not shown because there are no active oxygen species on them.
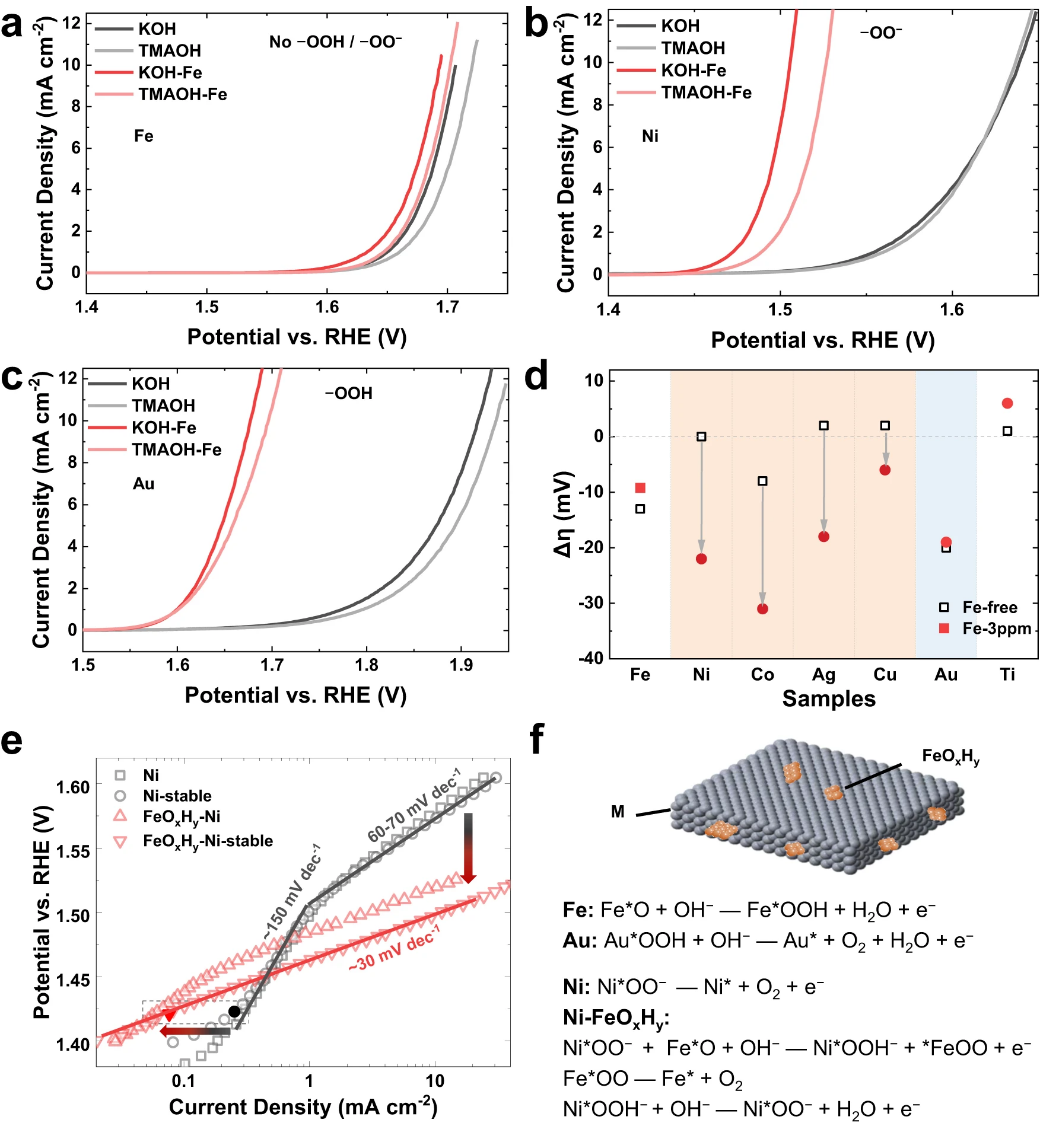
Figure 4. Synergistic interaction between FeOxHy and superoxide groups (-OO−). (a, b, c) Polarization curves of (a) Fe, (b) Ni, and (c) Au foils in 1 M KOH and 1 M TMAOH with or without the addition of 3ppm Fe. (d) Potential degradation in the electrolyte transition from 1 M KOH to 1 M TMAOH. The samples with superoxide groups (–OO−) are highlighted with an orange background and the one with peroxyl group (–OOH) is highlighted with a blue background. (e) Tafel plotting of Ni foams in Fe-free and Fe-sparking KOH. To clarify the transition, both the initial and the stable ones are present. (f) Structural model of FeOxHy on metal foils (M(O)OH) and the proposed catalytic mechanism.
Conclusion
In summary, this work uncovers that the promoting effect of FeOxHy to other transition metal oxyhydroxides is triggered by the interaction with active oxygen species. The OER catalytic activities and Tafel slopes exhibit strong dependence on the emergence and kinds of oxygen species in FeOxHy @M(O)OH (M=Fe, Co, Ni, Cu, Ag, Au, Ti, Nb, and Sn). FeOxHy synergistically promotes the substrates that can produce active oxygen species exclusively. More importantly, the onset OER potentials of FeOxHy @M(O)OH coincide with the emerging potentials of active oxygen species, whereas dozens or hundreds of millivolts’ potential gaps are present for intact MOOH. This correlation implies a strong synergy between the anodic deposited FeOxHy and the active oxygen species of the MOOH substrate and was further validated by the chemical probe experiments. Proton transfer/diffusion was suggested to be essential to the OER catalysis of FeOxHy -promoted M(O)OH.
Corresponding Authors
Fang Song: Associate Professor, Doctoral Supervisor of State Key Laboratory of Metal Matrix Composites at Shanghai Jiao Tong University. Research topics: 1. Electrocatalysts for water splitting; 2. Bio-inspired and Biomimetic Materials.
