Background
Sodium-ion batteries (SIBs) are viewed as promising alternatives to lithium-ion batteries due to the advantages of abundant sodium resources and high safety. At the moment, improving the energy density and fast charging properties of SIBs is critical to promote their rapid development. Molybdenum disulfide (2H phase MoS2) with a unique layered structure has a high theoretical capacity, however, its rate performance and redox reversibility are typically poor due to the slow charge transfer mediated by polarons. Traditional modification strategies are often centered on the construction of nano-size/pore-controlled carbon composite structures. Despite improvements in electrochemical performance, the low volume energy density and elaborate manufacture hinder its practical application. Although phase transition engineering can create conductive 1T phase MoS2, the metastable property causes it to re-stack to the stable 2H phase throughout the cycle process, which inevitably reduces the electrochemical performance. Therefore, it remains a significant challenge to efficiently build a thermodynamically stable molybdenum sulfide anode with a high rate characteristic and extended cycling lifespan by rational crystal structure design of MoS2.
Introduction
Prof. Fuqiang Huang, from Shanghai Jiao Tong University, proposed a novel polaron collapse strategy to obtain a fast-charging Mo2S3 anode. Specifically, a thermodynamically stable metallic Mo2S3 was designed by inserting 1D [MoS] chains into the 2D MoS2 layers through a solid-state reaction. DFT calculations with an excess electron injection reveal that small polaron in MoS2 (−0.9 V vs. Fermi level) disappears after the insertion of [MoS] chains that break the symmetry of 2D layers in the c-direction. As-induced small-polaron collapse in Mo2S3 liberates electrons from the self-trapped state and thus delocalized electron state will be achieved. This accounts for a 107 times higher electrical conductivity in Mo2S3 (4.4×104 S m−1), compared with that in 2H-MoS2 (4.5×10−3 S m−1). Further theoretical calculations confirm that Mo2S3 owns highly delocalized anions, which substantially reduces the interactions of Na−S to kinetically accelerate Na+ diffusion in crystal material with a much lower energy barrier (0.38 vs 0.65 eV of MoS2). The Mo2S3 anode exhibits a high capacity of 510 mAh g−1 at 0.5 C, and superior high-rate stability of 310 mAh g−1 at 20 C over 5000 cycles as well as 217 mAh g−1 at 40 C over 15000 cycles, outperforming previously reported advanced SIB anodes. Furthermore, the potentiality of Mo2S3 in flexible electronics has been exhibited. This work demonstrates that polaron engineering could obtain intrinsic accelerated electron/ion transport, which is expected to supplement an effective electrode design strategy for fast-charging batteries. The relevant article, entitled “1D Insertion Chains Induced Small-Polaron Collapse in MoS2 2D Layers Toward Fast-Charging Sodium-Ion Batteries” was published in Advanced Materials.

Paper link
https://onlinelibrary.wiley.com/doi/10.1002/adma.202309637
Research content
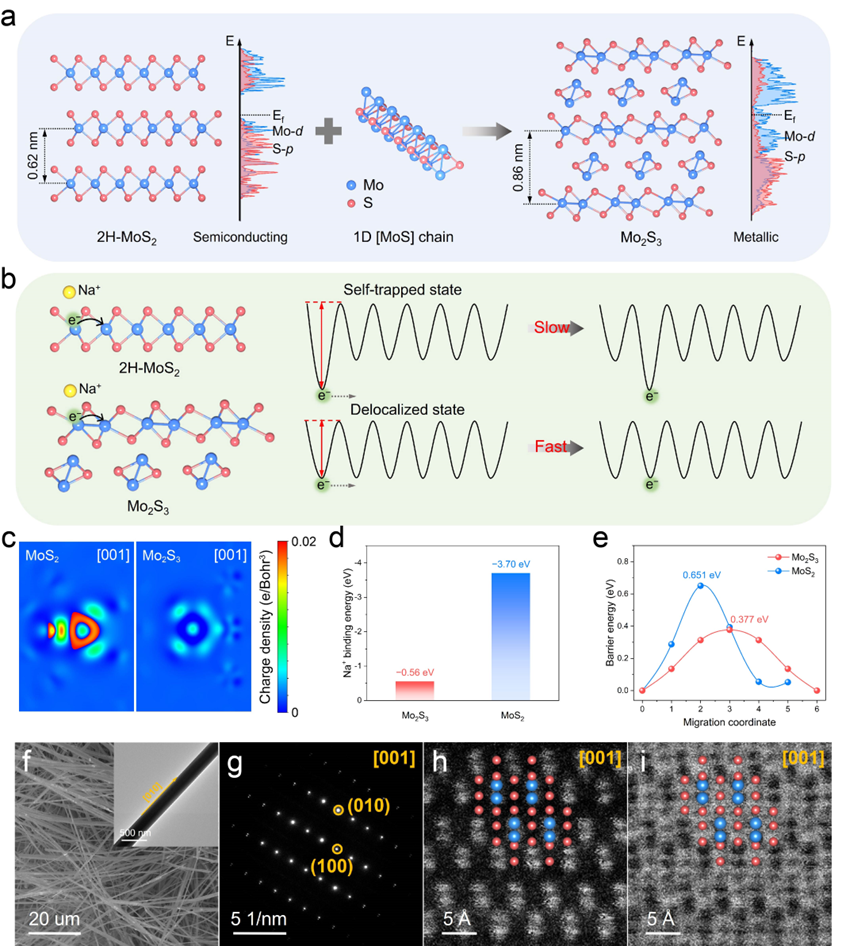
Figure 1. Design schematic, theoretical calculations, and crystal structure of Mo2S3. a) Structure design of Mo2S3 from 2D MoS2 layer and 1D [MoS] chain. b) Schematic illustration of excess electron state in different potential wells. c) Charge density difference maps from the [001] direction, d) Na+ binding energies, and e) Na+ diffusion barrier energies of MoS2 and Mo2S3. f) SEM image and TEM image (the inset), g) SEAD pattern, h) ADF-STEM image, and i) ABF-STEM image of Mo2S3.
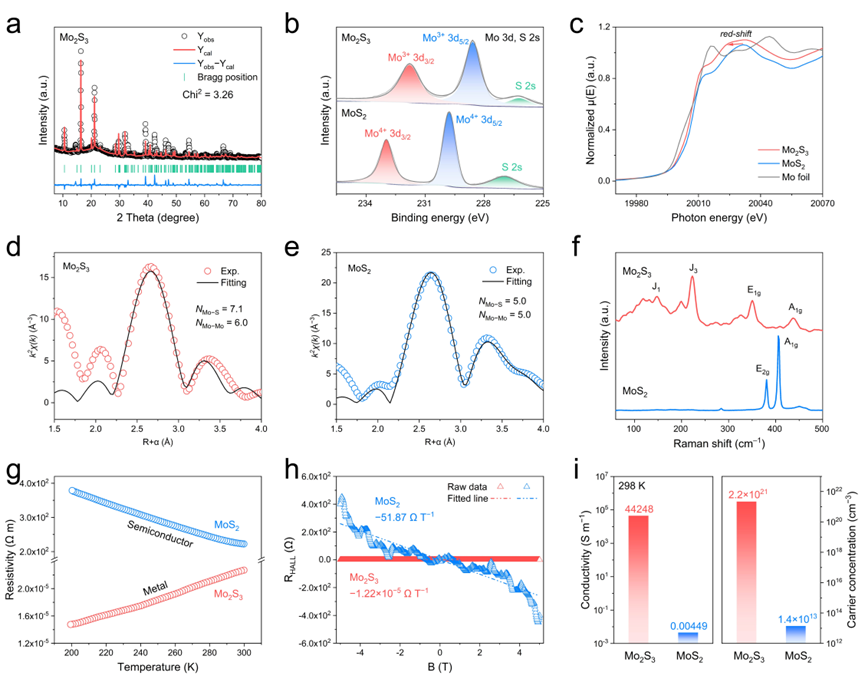
Figure 2. Material characterizations. a) Rietveld refined XRD pattern of Mo2S3. b) XPS spectra of Mo 3d, and c) Normalized Mo K-edge XANES spectra of Mo2S3 and MoS2. Mo K-edge FT-EXAFS spectrum (with phase corrections) of d) Mo2S3 and e) MoS2 and the corresponding fitting curves in the first coordination shell. f) Raman spectra, g) Temperature-dependent resistivity curves, h) Magnetic flux density-dependent Hall resistivity curves, and i) Electrical conductivities and carrier concentrations at room temperature of Mo2S3 and MoS2.
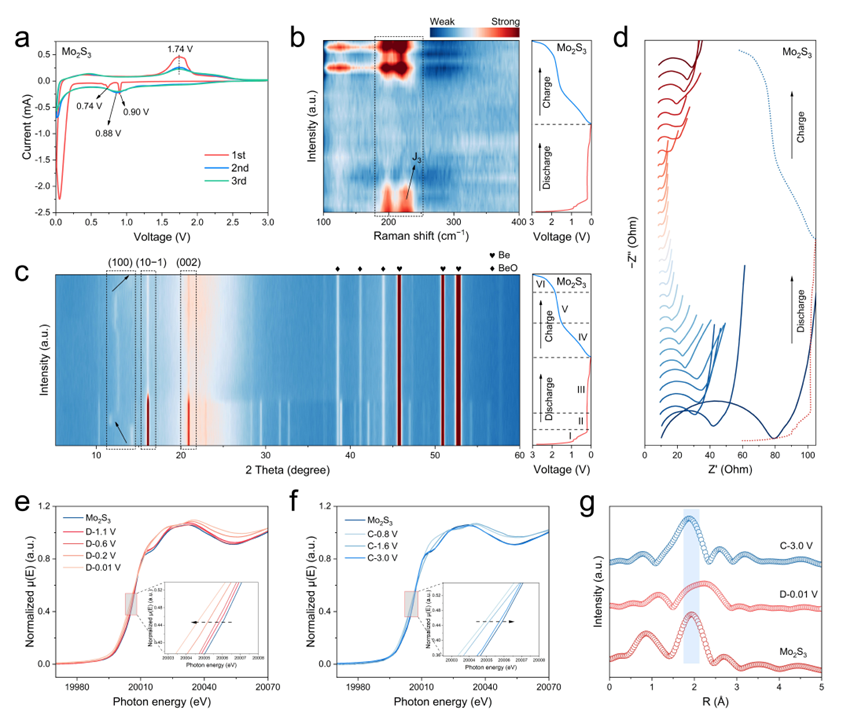
Figure 3. Structure evolution upon sodiation and desodiation. a) CV curves of Mo2S3 at 0.2 mV s−1 in the initial three cycles. b) In situ Raman spectra, c) In situ XRD patterns, and d) In situ Nyquist plots of Mo2S3 in the first cycle. Ex situ Mo K-edge XANES spectra of Mo2S3 during the first e) discharged and f) charged states. g) Ex situ Mo K-edge EXAFS spectra (without phase correction) of Mo2S3 at pristine, fully discharged, and fully charged states.
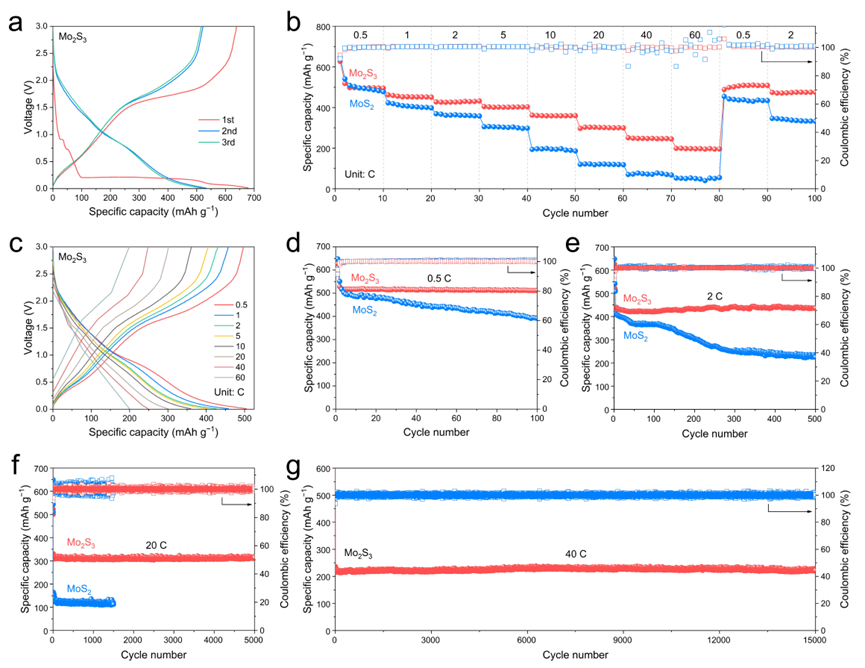
Figure 4. Electrochemical performance. a) GCD curves of Mo2S3 in the initial three cycles. b) Rate capability of Mo2S3. c) GCD curves of Mo2S3 at different current densities. Cycling performance of Mo2S3 at current densities of d) 0.5 C, e) 2 C, f) 20 C, and g) 40 C.
Conclusions
To summarize, ultrahigh-rate and robust sodium storage performance has been achieved via chemically integrating 2D MoS2 layers and 1D [MoS] chains. The insertion of 1D [MoS] chains breaks the symmetry states of 2D MoS2 layers, inducing small-polaron collapse, which substantially enhances the electron/ion transport in crystal material. The results were confirmed by DFT calculations. The as-obtained Mo2S3 anode presents superior rate capability and stability for maintaining 217 mAh g−1 at 40 C after 15000 cycles, and the application in flexible electronics has been demonstrated. The proposed polaron collapse strategy induced by electron-rich insertions should make sense for high-rate electrode design.
Corresponding authors
Fuqiang Huang: Professor, State Key Lab of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University.
Ximeng Lv: Post-doctor, Laboratory of Advanced Materials, Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University.
