Recently, Professors Jianbo Wu and Wenpei Gao from the School of Materials Science and Engineering at Shanghai Jiao Tong University have published a significant research article titled "Direct In-Situ Imaging of Electrochemical Corrosion of Pd-Pt Core-Shell Electrocatalysts" (DOI: https://doi.org/10.1038/s41467-024-49434-3) in Nature Communications (Impact Factor: 16.6). The first authors of the study are PhD students Fenglei Shi and Hao Hu, along with Peter Tieu from the University of California, Irvine. Professors Jianbo Wu, Wenpei Gao, and Xiaoqing Pan (University of California, Irvine) are the co-corresponding authors. This research was also supported by Prof. Tao Deng, Wen Shang, Chengyi Song, Peng Tao, and other collaborators from SJTU.
In electrochemical redox processes, long-term cycling inevitably leads to corrosion and the subsequent deactivation of electrocatalysts. Therefore, exploring the electrochemical corrosion mechanism of catalysts is crucial for guiding the design of high-performance electrocatalysts. With the continuous development of micro- and nanofabrication technologies in recent years, the successful integration of liquid-phase in situ transmission electron microscopy (TEM) with electrochemical systems has made it possible to investigate the electrochemical corrosion process under working conditions.
However, studying the correlation between the structural evolution of nanoparticles and the electric potential requires higher resolution of the TEM and precise acquisition of electrochemical signals. Previously, Professor Jianbo Wu used liquid-phase in situ TEM to simulate corrosion at high potentials through the oxidative corrosion of palladium atoms by Br ions. This revealed the effects of defects, strain, and local curvature in the microscopic corrosion of palladium-platinum core-shell nanoparticles at the nanoscale (Nat. Commun. 2018, 9, 1011; Chem 2020, 6, 2257-2271). However, there is still a significant difference between this direct chemical corrosion and actual electrochemical corrosion, highlighting the importance of studying the corrosion of nanomaterials in a more realistic electrochemical environment.
To address this challenge, the research team introduced electrochemical signals into liquid-phase in situ TEM. This approach not only accurately recorded complete cyclic voltammetry (CV) curves with clear redox peaks under in situ conditions but also revealed that the surface reconstruction occurring in nanoparticles during the redox process is driven by the cyclic recurrence of oxidative solvation and redeposition of palladium atoms. This work lays a foundation for the study of the electrochemical corrosion mechanisms of catalysts.
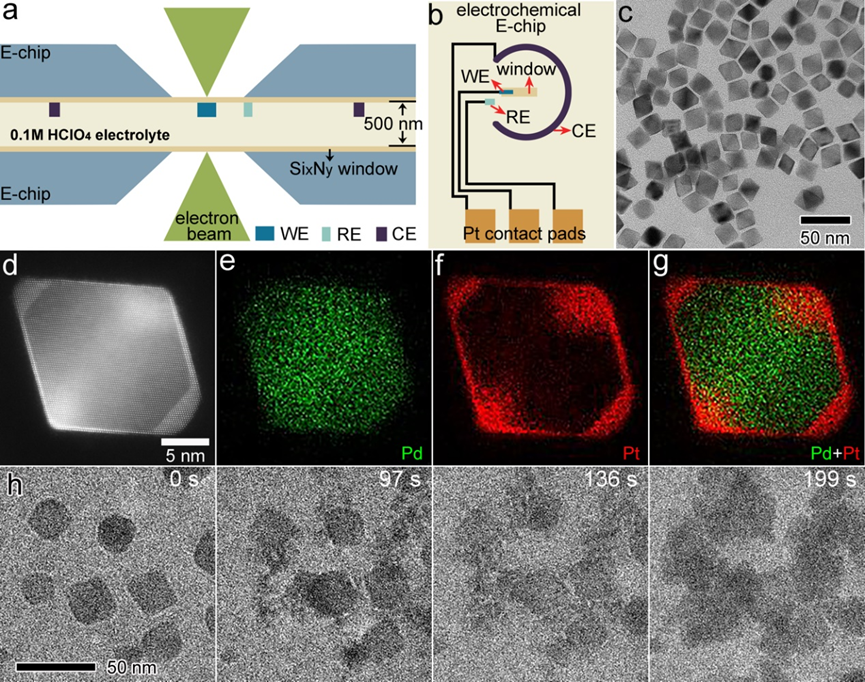
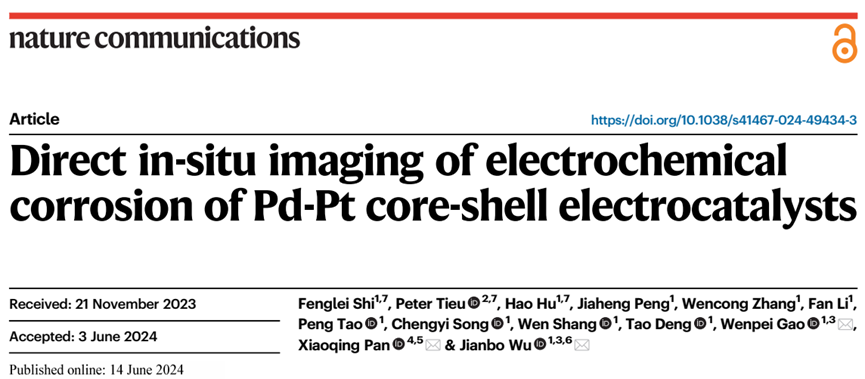
Figure 1: (a) Schematic diagram of an electrochemical liquid cell. (b) Schematic illustration of the electrochemical E-chip. (c-g) TEM image and EDS mapping of Pd@Pt octahedron. (h) in-situ TEM images of the fast electrochemical corrosion process.
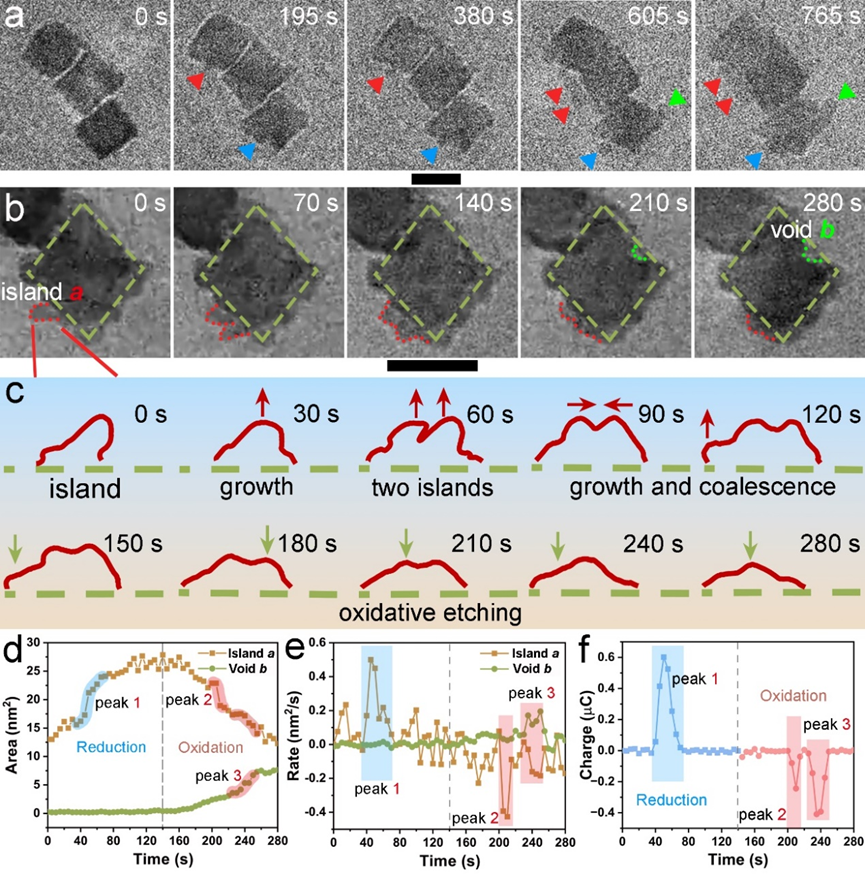
Figure 2: (a, b) in-situ TEM images of morphological evolution under different potential ranges. (c) Trajectory analysis of Island a in b. (d, e) Area changes and rates of Island a and Void b. (f) charge of the corresponding redox peaks in CV.
Figure 3: (a) in-situ CV curve. (b) The adjacent CV curves showing the dynamic of the electrochemical corrosion process. (c) Thematic illustration of the oxidation potential difference between surface Pd, Pt, and inner Pd atoms.
The research team synthesized Pd-Pt core-shell octahedral nanoparticles for subsequent in-situ electrochemical corrosion experiments (Figure 1). They discovered that high voltage intervals led to excessive corrosion rates, so they selected appropriate potential ranges for corrosion kinetic analysis at the nanoscale through potential modulation. Interestingly, Pt located on the terraces corroded preferentially compared to Pt on the corners and edges due to adsorption, which is different from previously observed corrosion phenomena.
Additionally, the electrochemical corrosion process of individual nanoparticles was analyzed in detail (Figures 2, 3). During the redox process, the nanoparticle surface underwent a reconstruction phenomenon primarily due to the oxidative corrosion and reductive deposition of palladium atoms. The specific process includes the preferential corrosion of external palladium atoms, followed by the corrosion of a small amount of low-coordinated platinum, and then the corrosion of the predominantly internal palladium atoms, with subsequent palladium redeposition. Based on these observations, the researchers proposed a corrosion model for the dissolution and redeposition of metal atoms during high and low potential cycling.
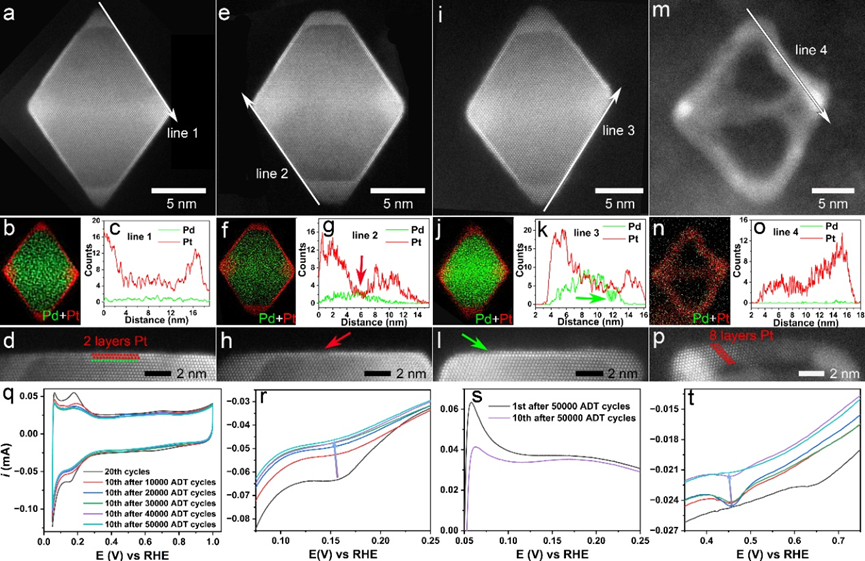
Figure 4: HAADF-STEM characterization and CVs of Pd@Pt octahedron electrocatalysts after ex-situ electrochemical ADTs.
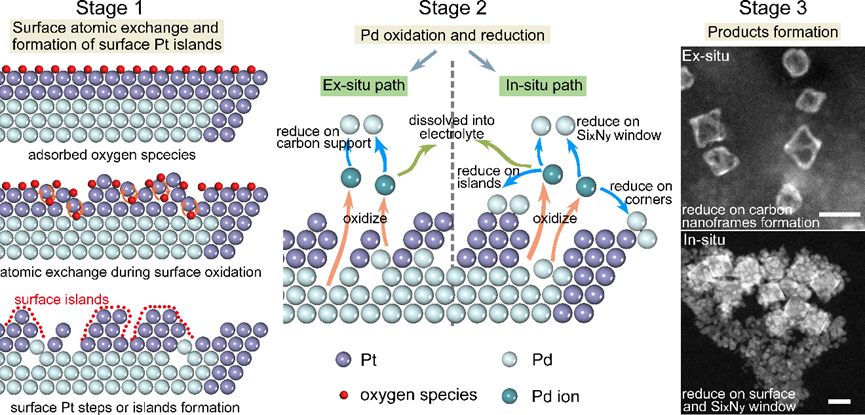
Figure 5: Schematic diagram of the mechanisms of electrochemical corrosion via ex-situ and in-situ paths, respectively.
The research team also conducted real electrochemical stability tests (Figure 4) to further reveal the corrosion behavior of core-shell nanoparticles under actual electrochemical conditions. They ultimately summarized the electrochemical corrosion mechanisms under both in-situ and ex-situ conditions (Figure 5).
This study reveals the corrosion kinetics of nanocatalysts under electrochemical conditions, providing valuable guidance for the synthesis of catalysts with higher stability and application value. It also contributes significantly to the understanding of the electrochemical fundamentals in catalytic reactions at the atomic level.
This work was supported by the National Key R&D Program of China, the National Science Foundation of China, the Program of Shanghai Academic/Technology Research Leader, the Innovation Program of Shanghai Municipal Education Commission, the 111 Project, and the support from Center of Hydrogen Science and Joint Research Center for Clean Energy Materials from Shanghai Jiao Tong University.
Read the full article:https://www.nature.com/articles/s41467-024-49434-3
