Recently, the world’s first 3D printed vertebral fusion device based on metamaterials, developed by Professor Jianfeng Gu’s team from the School of Materials Science and Engineering at Shanghai Jiao Tong University, along with Dr. Haozhang Zhong, successfully obtained 510(k) clearance from the U.S. Food and Drug Administration (FDA), with FDA number K232996 (Figure 1). This marks a significant step for this innovative technology in the medical device field. This is the first artificial bone implant designed based on metamaterial principles, and its breakthrough technology not only demonstrates the application potential of metamaterials in medicine but also sets a new milestone for the development of orthopedic implants.
Since early 2021, the team has been applying metamaterial technology to the design and development of vertebral fusion devices (i.e., artificial bones), aiming to enhance mechanical compatibility, improve the interaction between the implant and the patient’s bone, and boost the bio-integration performance of the implant. After three and a half years of relentless effort, the team successfully developed a vertebral fusion device with excellent mechanical properties and biological adaptability, ultimately receiving FDA 510(k) certification.
Product Features and Technical Innovations
The certified vertebral fusion device encompasses various models and sizes (Figure 2) suitable for surgical approaches such as ALIF, TLIF, and LLIF, primarily used to treat common orthopedic conditions such as herniated discs, vertebral fractures, and vertebral tumors (Figure 3). The innovative design of the product integrates multiple cutting-edge technologies, particularly the design concept of its metamaterial building blocks (Gu et al., Materials Today, 2023), which maximizes porosity while maintaining necessary strength, thus promoting bone tissue integration and ingrowth.
Additionally, the key physical properties of the implant, including density (1.6 g/cm³), Young's modulus (13.6 GPa), and strength, are highly compatible with human bone, effectively enhancing biological adaptability. Meanwhile, the contour of the fusion device employs the team's developed conformal design technology (Gu et al., Current Opinion in Solid State and Materials Science, 2024), achieving precise shaping of the metamaterials and structural conformity of the implant, further enhancing the functional performance of the implant.
Research Achievements and Intellectual Property
As the world’s first bone implant designed and manufactured using metamaterial technology, this product has generated theoretical breakthroughs and innovative technologies throughout its development. These advancements have been published in several high-level research papers and have secured multiple patent protections. To date, the related research findings include 10 published papers in SCI journals, along with 5 Chinese invention patents, 2 U.S. invention patents, and 5 utility model patents, as well as 4 software copyrights. These intellectual properties lay a solid foundation for the product's future promotion and further development.
Outlook
The FDA certification of this vertebral fusion device marks a new application phase for the metamaterial research conducted by the material modification and numerical simulation team. The team will continue to deepen the application of metamaterial technology in the field of biomedical materials, driving more innovative products to market and benefiting a greater number of patients. In the future, as metamaterial technology advances, more medical products based on this technology are expected to enter clinical use, fostering innovation and development within the medical device industry.

Figure 1. FDA Certification Letter from the U.S. Food and Drug Administration.
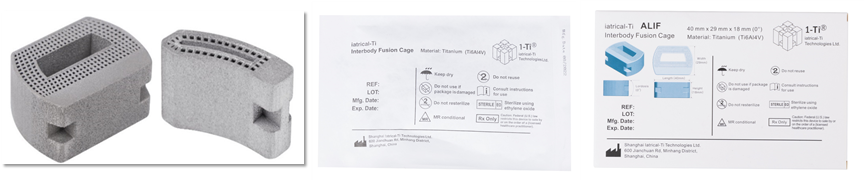
Figure 2. Display of the Fusion Device Product and Packaging Box.
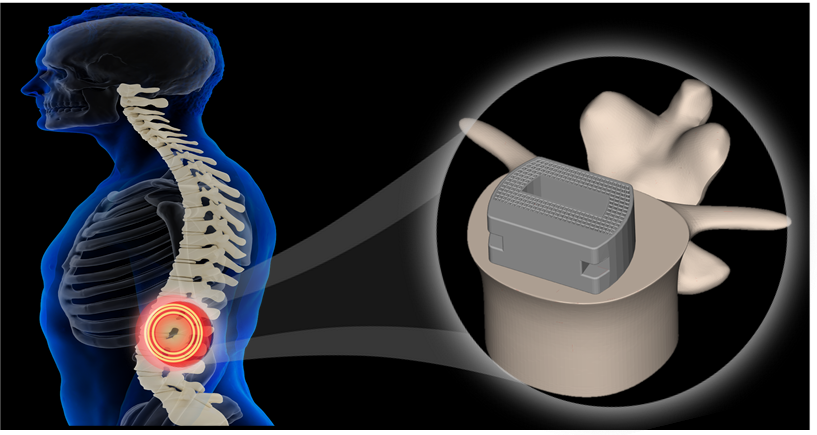
Figure 3. Schematic Illustration of the Fusion Device for Lumbar Spine Use in Treating Conditions such as Herniated Discs.
