Researchers at Shanghai Jiao Tong University have developed a groundbreaking method to enhance the stability and activity of PtFe nanowire catalysts for proton exchange membrane fuel cells (PEMFCs). Their innovative surface atomic ordering strategy significantly improves catalytic performance and durability, addressing key challenges in fuel cell technology. The study, titled “Highly Stable and Active Catalyst in Fuel Cells Through Surface Atomic Ordering” (DOI: 10.1126/sciadv.ado4935), was published in Science Advances with Shanghai Jiao Tong University as the lead institution.
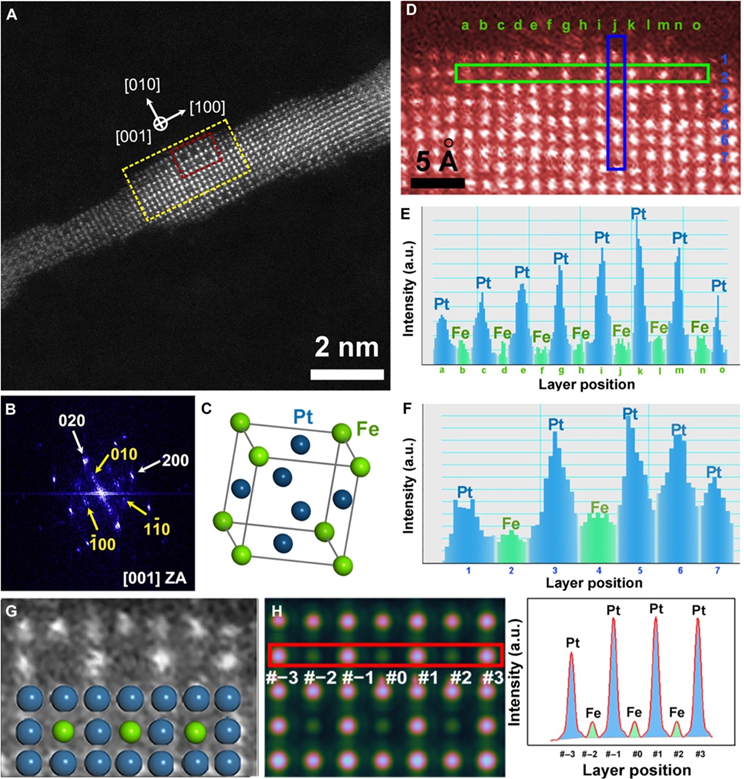
Intermetallic surface formation on PtFe NWs under in situ low-temperature annealing.
The slow oxygen reduction reaction (ORR) at the PEMFC cathode is a bottleneck that limits efficiency, power density, and durability. Although shape-controlled Pt-based alloy nanocrystals have demonstrated excellent catalytic activity in half-cell tests, their stability in practical fuel cells is often compromised under harsh operating conditions in membrane electrode assemblies (MEAs), such as high temperatures, potential cycling, and acidic environments. These conditions accelerate Fe dissolution, degrade the catalyst, and cause metal ions to accumulate near the surface, hindering proton transport. Enhancing the stability of PtFe alloy catalysts while maintaining high activity is crucial for their effective deployment in PEMFCs.
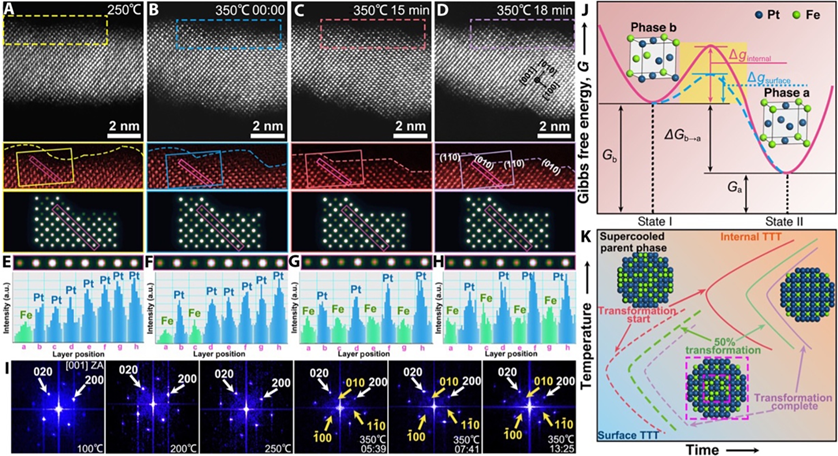
Disorder-to-order transformation of the PtFe NW surface structure
The researchers achieved surface atomic ordering in PtFe nanowires with atomic precision by using in-situ scanning transmission electron microscopy (STEM) to observe the transition from a disordered to an ordered phase. By annealing 3nm PtFe nanowires at 350°C for no more than 30 minutes, they induced surface ordering into the Ll2-Pt3Fe phase while preserving the one-dimensional morphology. This low-temperature surface atomic ordering (LT-SAO) strategy successfully prevented aggregation or breakage of the nanowires. Surface ordering was confined to three atomic layers, ensuring structural stability in the interior while avoiding excessive phase transformation that could degrade the nanowires.
The surface-ordered PtFe nanowires demonstrated significant improvements in catalytic performance and durability. Their mass activity (MA) in MEA tests was double that of disordered PtFe nanowires. They retained 78.6% of their initial MA after 30,000 cycles of durability testing, far exceeding the durability target of an MA loss below 40%. Furthermore, the corrosion resistance of the surface-ordered nanowires was markedly enhanced, with Fe dissolution reduced to 41.3%, compared to 94.1% for disordered nanowires. These results establish the effectiveness of surface atomic ordering in stabilizing catalysts under real-world conditions.
To further understand the enhanced stability of the surface-ordered catalysts, the team conducted density functional theory (DFT) calculations on atomic models of ordered Ll,2-Pt3Fe and disordered Al-PtFe phases. The calculations revealed that the Ll,2-Pt3Fe structure provides much improved resistance to galvanic corrosion, significantly reducing Fe leaching and mitigating catalyst degradation. This improvement stems from the formation of an intermetallic surface, which enhances the catalyst’s stability in acidic environments, offering a theoretical foundation for the experimental observations.
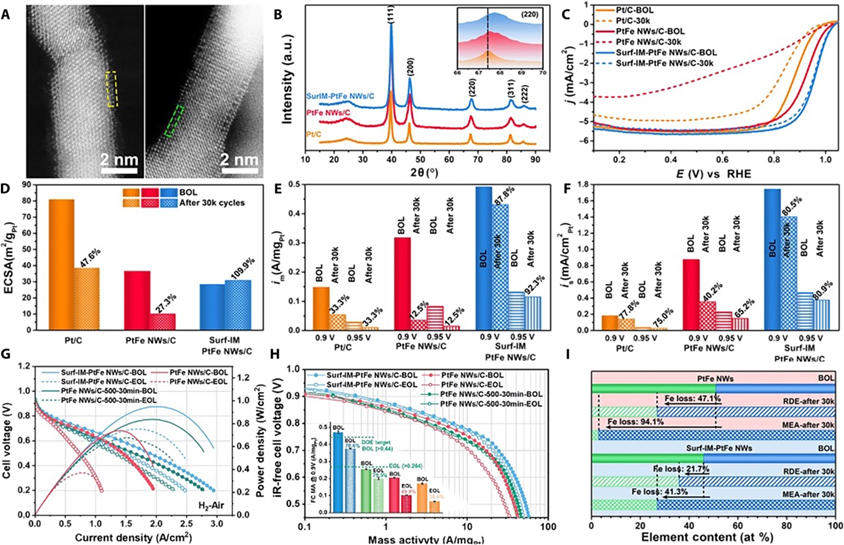
Electrocatalytic activity and durability evaluations in a liquid half-cell and single fuel cell
This study represents a significant advancement in the design principles for high-performance, durable fuel cell catalysts. The surface atomic ordering strategy not only enhances the ORR activity and stability of PtFe nanowires but also demonstrates potential applicability to other Pt-based alloy nanocrystals. By addressing both scientific and technological challenges, this work paves the way for sustainable, high-efficiency PEMFCs and supports the broader transition to clean energy solutions.
The collaborative research team includes Professors Jianbo Wu, Wenpei Gao, and Tao Deng from the School of Materials Science and Engineering, Hydrogen Science Center, and the State Key Laboratory of Metal Matrix Composites at Shanghai Jiao Tong University, and Professor Xiaoqing Pan from the University of California, Irvine. The research was supported by National Key R&D Projects, the National Natural Science Foundation of China, the Shanghai Academic/Technical Leader Project, the Shanghai Municipal Education Commission Innovation Projects, and key research centers at Shanghai Jiao Tong University, including the Hydrogen Science Center, Materials Genome Joint Research Center, and Clean Energy Joint Research Center. The contributions of these organizations and collaborators were instrumental in enabling this groundbreaking work.
