Recently, Professor Jin Xuejun and Dr. Xu Yuantao from the School of Materials Science and Engineering at Shanghai Jiao Tong University, in collaboration with teams led by Academician Sun Jun and Professor Liu Gang from Xi'an Jiaotong University, Professor B. Gault from the Max-Planck Institute for Sustainable Materials in Germany, and Associate Professor Wang Ruihong from Xi'an University of Technology, as well as other domestic and international teams, have made significant progress in the development of new hydrogen-resistant aluminum alloys. The relevant research results, titled "Structurally complex phase engineering enables hydrogen-tolerant Al alloys," were published online in Nature. This work achieved the controllable phase transformation construction of complex-structured hydrogen storage particles, demonstrating an effective way to overcome the contradiction between "strength and hydrogen embrittlement sensitivity" in high-strength aluminum alloys. Under the condition of hydrogen charging up to 7 ppmw, excellent tensile uniform elongation was obtained. The strategy of growing nanoscale complex intermetallic phases on nano-strengthening phases laid a theoretical foundation for the development of advanced hydrogen-tolerant aluminum alloys with higher strength. Jiang Shengyu, a doctoral student at Xi'an Jiaotong University, is the first author of the article. Dr. Xu Yuantao from Shanghai Jiao Tong University and Associate Professor Wang Ruihong from Xi'an University of Technology are the co-first authors. The co-authors of the article also include Dr. Liu Fuzhu from Xi'an Jiaotong University, Professor B. Gault and Dr. X. R. Chen from the Max Planck Institute for Renewable Energy Research in Germany, Dr. Guan Chaoshuai and Professor Peng Yong from Lanzhou University, the team led by Professor Wang Huiyuan from Hebei University of Technology, Professor Jin Xuejun from Shanghai Jiao Tong University, Dr. Tian Genqi from Shanghai Power Equipment Research Institute, Professor H. Toda from Kyushu University in Japan, and Dr. Wang Mingxu from Shandong University. Dr. Xu Yuantao, Professor Liu Gang, Professor B. Gault, and Academician Sun Jun are the co-corresponding authors of the article.
Aluminum alloys are widely used in fields such as marine, aerospace, and hydrogen energy storage and transportation. During the development and application of lightweight aluminum alloys, stress corrosion cracking (SCC) and hydrogen embrittlement have been major problems throughout the entire application history of aluminum alloys. The SCC behavior is mainly caused by the enrichment of hydrogen at stress concentration sites, and anodic dissolution only promotes hydrogen embrittlement. How to weaken or delay the hydrogen embrittlement problem of aluminum alloys during their use has become a major challenge in the application of aluminum alloys. Since the occurrence of hydrogen embrittlement depends crucially on the hydrogen content and its diffusion within the microstructure, regardless of the specific hydrogen embrittlement mechanism, preventing hydrogen from entering and introducing deep hydrogen traps to reduce the hydrogen diffusion rate are usually effective. Based on this, many methods have been developed, such as introducing intermetallic compound (IMC) particles as effective media for hydrogen capture and storage. However, common high-density nano-strengthening phases in aluminum alloys, such as Al₂Cu, Al₃Sc, Mg₂Si, and MgZn₂, have low hydrogen trapping capabilities, or hydrogen absorption at their interfaces is likely to lead to interfacial delamination and cracking. IMC particles with high hydrogen trapping capabilities, such as Al₁₁Mn₃Zn₂, Al₇Cu₂Fe, Al₆Mn, and Al₇Cr, often have a very low number density. It is difficult to achieve both high number density and high hydrogen trapping capabilities of hydrogen storage particles. Therefore, how to tailor and prepare nanoscale IMC particles with both high hydrogen trapping capabilities and high-density, dispersed distribution characteristics is a scientific and technical problem in the aluminum alloys.
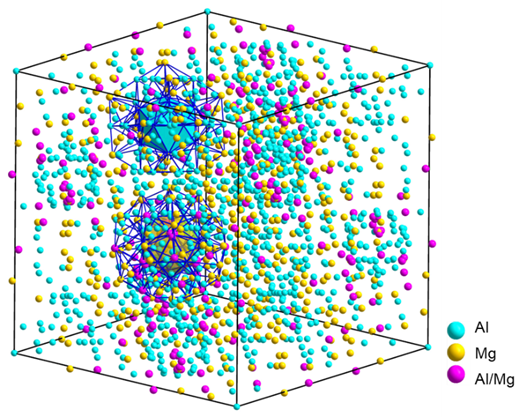
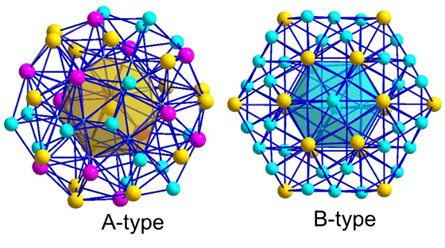
Figure 1 Giant-unit-cell complex metallic phase. Schematic diagram of the crystal structure of the Samson - Al₃Mg₂ complex metallic phase unit cell (top) and the atomic cluster model diagram for its structural description (bottom: Type A and Type B atomic clusters). The pink positions in the figure represent sites that can be randomly occupied by Al or Mg.
In response to the challenges faced in the development of hydrogen-resistant aluminum alloys, the joint research team conducted systematic research. They found that complex metallic phases (CMPs) are a special type of intermetallic compound with characteristics such as icosahedral coordination, a large number of atoms in the unit cell, and a large lattice constant. The Samson phase Al₃Mg₂, as one of the most complex CMPs, has only 1,168 Al or Mg atoms occupying its 1,832 lattice sites (Figure 1). Nearly 40% of the sites are structurally disordered or have structural vacancies, making it a natural and excellent hydrogen traps. First-principles calculations show that its hydrogen binding energy (Eb) is greater than 0.9 eV/atom, exceeding all reported second-phase particles in aluminum alloys (Figure 2). However, the Samson - Al₃Mg₂ phase has a high nucleation energy barrier. It usually nucleates non-uniformly at high-energy positions such as grain boundaries and coarsens into micron-scale particles, making it difficult to form a high-density and dispersed distribution within the grains. Its bottleneck problem is similar to that of crystalline phase particles. Therefore, the nano-scale controllable precipitation of the Samson - Al₃Mg₂ phase is the fundamental way to solve this problem and an effective approach for the engineering development of new hydrogen-resistant aluminum alloys.
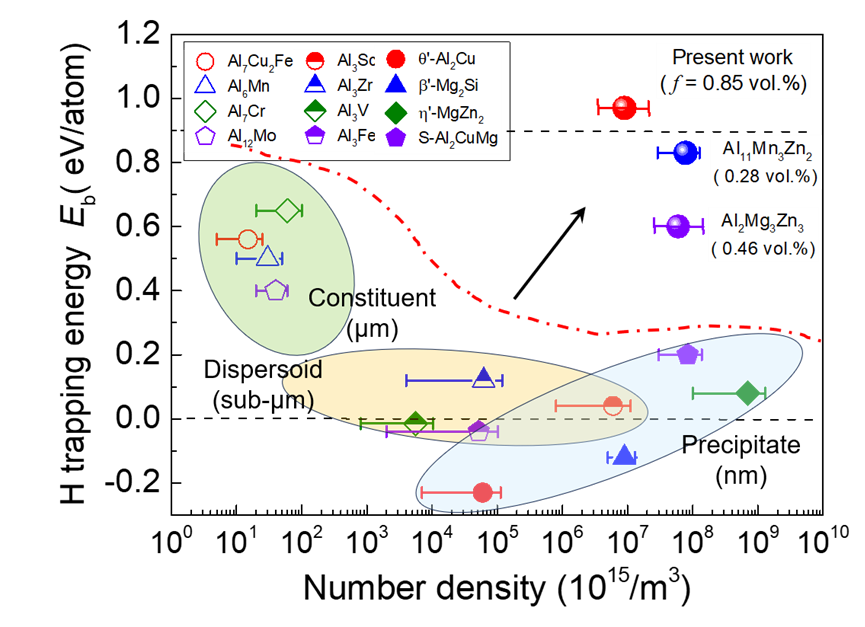
Figure 2 Unprecedented H-trapping ability of the complex metallic nanophase. Intermetallic compound particles (ICPs) in Al alloys: trade-off relationship between H-trapping energy (Eb) and number density, two requisites for mitigating HE resistance. The ICPs that are predicted to have a high Eb normally exists as coarse constituent particles, in μm-scaled size and a low number density typically < 1018/m3. The ICPs that could be precipitated as high-density, nanosized precipitates (> 1020/m3) normally have a low Eb. The sub-μm-sized dispersoids generally exhibit a low number density and a low Eb. The exceptions are the newly-found Al11Mn3Zn2 and Al2Mg3Zn3 that show a high Eb and a high number density, but in a low volume fraction of only ~ 0.28 vol.% and ~ 0.46 vol.%, respectively. The Al3(Mg,Sc)2 nanoprecipitates designed in this paper has the largest reported Eb, a number density equivalent to that of the traditional nanoprecipitates, and a volume percentage, which means a stronger hydrogen trap and endows it with a more excellent hydrogen embrittlement resistance.
The joint team worked closely together. In an Al - Mg (Mg content 4.5 - 7.5 wt.%) alloy with a small amount of Sc added, a dual-stage precipitation system induced by two-step heat treatment was designed. Through the control of complex phase transformations depending on the size of the nanoprecipitates in the Al - Mg alloy with Sc added, a high-density and dispersed distribution of Al₃Sc nano-precipitation phases was achieved. This not only maintained its strengthening effect but also in-situ formed a core-shell Al₃(Mg,Sc)₂/Al₃Sc nanoprecipitates with unprecedentedly high hydrogen trapping capabilities at its interface. After the two-step heat treatment, the dispersed Samson phase - Al₃(Mg,Sc)₂ within the grains preferentially nucleated non-uniformly on the surface of Al₃Sc with a size of about 10 nm, while the surface of Al₃Sc with a size below 10 nm remained unchanged (Figure 3). This proves that the size-dependent nucleation of nano Al₃(Mg,Sc)₂ is related to the coherency of the interface of nano Al₃Sc. The interfacial dislocations at the non-coherent interface will lead to local segregation of Mg, thereby triggering the formation of the complex phase Al₃(Mg,Sc)₂/Al₃Sc (Figure 4).
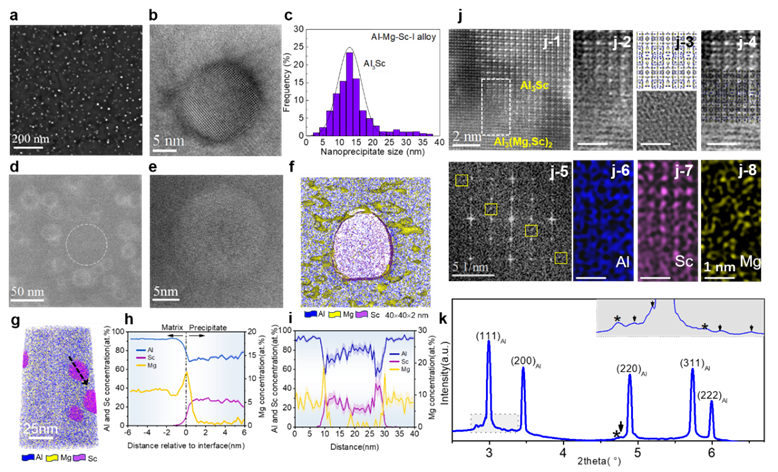
Figure 3 High-density Al3(Mg, Sc)2 nanophase by in situ phase transformation. (a - c) Low-magnification and high-resolution TEM images of the high-density Al₃Sc nanoprecipitates formed in the first heat treatment and their size statistical distribution diagram; (d, f) Low-magnification and high-resolution TEM images of the core-shell structured Al₃(Mg,Sc)₂/Al₃Sc nanoprecipitates formed in the second heat treatment; (f - i) Three-dimensional atom probe (APT) characterization of the high Mg content in the shell layer of the composite nanoprecipitates; (j, k) HAADF-STEM and synchrotron radiation characterization techniques to determine the Samson phase structure of the Al₃(Mg,Sc)₂ shell layer.
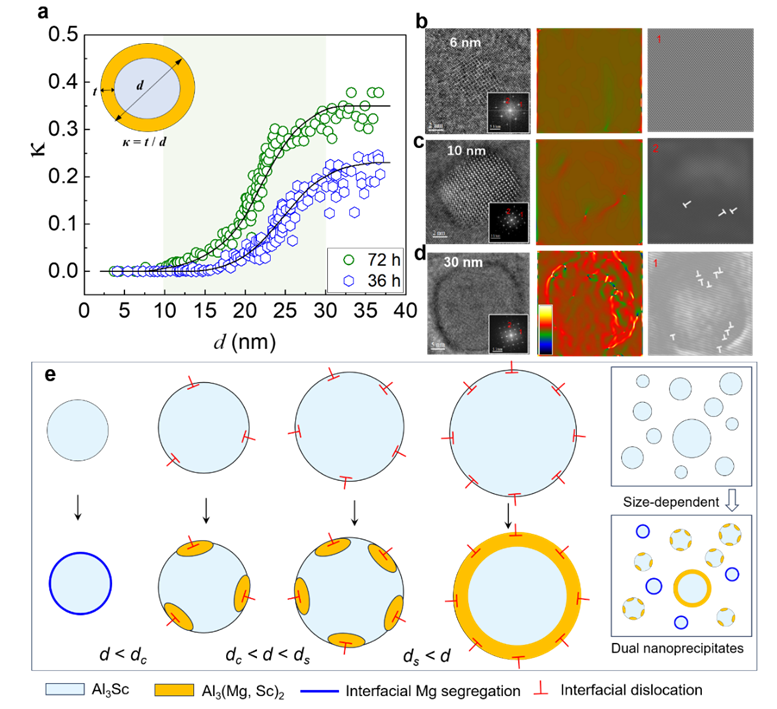
Figure 4 Interface-dominated in situ phase transformation. (a) Statistical results of the proportion κ of the Al₃(Mg,Sc)₂ nanophase changing with the size d of the Al₃Sc precipitates; (b - d) Local stress/strain field analysis and interfacial misfit dislocation characterization of Al₃Sc precipitates with different sizes. The larger the precipitate, the more interfacial misfit dislocations; (e) Schematic diagram of the in-situ phase transformation and its size effect, where dc is the critical size of Al₃Sc (about 10 nm) that induces the formation of Al₃(Mg,Sc)₂ by the template, and ds is the size of Al₃Sc (about 30 nm) with nearly complete coverage of the shell layer.
The tailored distribution of dual nanoprecipitates in our Al–Mg–Sc alloy provides about a 40% increase in strength and nearly five times improved HE resistance compared with the Sc-free alloy, reaching a record tensile uniform elongation in Al alloys charged with H up to 7 ppmw. The reduction in its tensile elongation was still less than 10%, and the tensile uniform elongation was greater than 10%, which is better than other reported aluminum alloy materials (Figure 5). In addition, this design of controllable phase transformation construction through complex precipitation overcame, to a certain extent, the contradiction between "strength and hydrogen embrittlement sensitivity" in aluminum alloys, achieving both strengthening and hydrogen embrittlement resistance. It also provided important reference for other structural materials sensitive to hydrogen embrittlement, such as high-strength steels and high-strength titanium alloys.
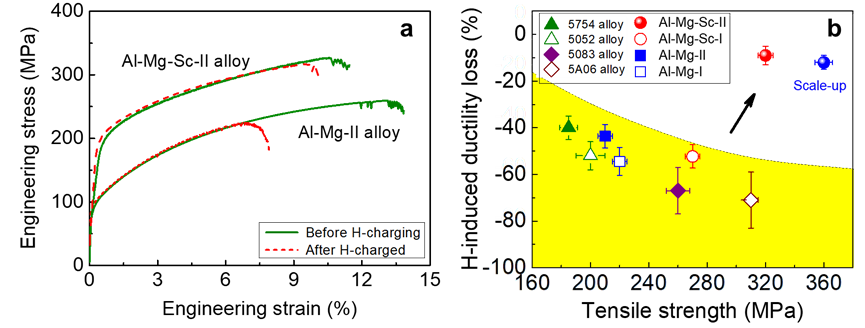
Figure 5 Extraordinary HE resistance boosted by the Samson-structured nanophase. (a) Comparison of the tensile stress-strain curves of Al - Mg - Sc and Al - Mg alloys before and after hydrogen charging at 7.0 ppmw: The elongation of the Sc-added alloy decreased by less than 10% after hydrogen charging, and the uniform elongation was as high as 10%; while the elongation of the Sc-free alloy decreased by more than 50% after hydrogen charging. (b) Relationship between the reduction in tensile elongation after hydrogen charging at 7.0 ppmw and tensile strength: The Al - Mg - Sc alloy (solid red dots) and its pilot test results (solid blue dots) in this study significantly surpassed 3 comparative alloys and 4 commercial Al - Mg series alloys (5754, 5052, 5083, 5A06).
The Hydrogen-Tolerant Structural Materials Research Team led by Professor Jin Xuejun at Shanghai Jiao Tong University has focused on the design and engineering application of high-strength structural materials for hydrogen-related environments in recent years. In the early stage, for the design and development of hydrogen-resistant high-strength aluminum alloys, the research team collaborated with teams led by Professor H. Toda and Associate Professor Wang Yafei from Kyushu University in Japan, and Professor B. Gault from the Max-Planck Institute for Sustainable Materials in Germany. They developed a variety of hydrogen-resistant high-strength aluminum alloy materials and published a series of articles in journals such as Nature Communications, Acta Materialia, and Corrosion Science. At present, China lags behind Japan and other countries in the research and development process of 70 - 140 MPa grade high-pressure gaseous hydrogen storage materials (steel/aluminum). The main problem is that high-pressure hydrogen storage materials face the "hydrogen embrittlement" challenge. Especially when the hydrogen pressure exceeds 100 MPa, extremely high requirements are placed on the hydrogen fatigue resistance of the materials themselves. The development of ultra-high-pressure hydrogen storage materials has high technical barriers and great production difficulties. It is also necessary to comprehensively consider forming, welding performance (hydrogen embrittlement performance of welded joints), and low-temperature toughness. Based on the design concept of multi-functional nanoprecipitates (precipitation strengthening and plasticizing, hydrogen storage), the research team and the Central Research Institute of Baosteel jointly tackled key problems and have initially developed hydrogen storage materials that meet the hydrogen pressure of 70 - 100 MPa. They are currently conducting an assessment of the hydrogen embrittlement performance of welded joints.
This research work was funded by projects such as "Research on the Hydrogen Embrittlement Mechanism of High-Pressure Gaseous Hydrogen Storage Materials" from the Central Research Institute of Baosteel and "Design and Development of High-Strength Aluminum Alloys Resistant to Stress Corrosion Cracking" from the Lanzhou Science and Technology Bureau. The article link address is: https://doi.org/10.1038/s41586-025-08879-2.
