
Chen et al.’s “An energy-saving pH-asymmetric electrolyzer for simultaneous methane conversion and hydrogen production” recently appeared in Science Bulletin. The research work focuses on developing a pH-asymmetric electrolyzer using waste-derived electrochemical neutralization energy enables low-energy methane conversion with hydrogen production. This work was conducted by Prof. Guo-Ming Weng’s team, with Prof. Zhenpeng Yao from the Zhangjiang Institute for Advanced Study (same university) providing advisory support for DFT calculations. Prof. Weng is currently an associate professor at the Shanghai Key Laboratory of Hydrogen Science & Center of Hydrogen Science, School of Materials Science and Engineering at Shanghai Jiao Tong University. The abstract appears below.
“A subsidiary role of electrochemical neutralization energy (ENE) stored in waste acid/base presents a sustainable and economically viable solution for the production of value-added chemicals and saving energy. Here we demonstrate a pH-asymmetric electrolyzer with a bipolar membrane to realize sustainable and efficient electrocatalytic methane oxidation coupling with hydrogen production at a low energy cost. This approach affords a C1/C2 molecule production rate of up to 2.6 mmol h–1 with simultaneous H2 production at a potential as low as 1.4 V. Furthermore, the electrocatalyst achieves sustained catalysis at 1 mA for over 24 h with lower operation voltage by 700 mV under auxiliary ENE supply, which means the cell electricity manner would enable an energy elimination of 36.9% energy saving comparing with state-of-the-art electrolyzers. In situ Raman spectroscopy and density functional theory calculation reveal the catalytic mechanism of the important role of NiIII–O∙–NiIII–O– for the electrochemical methane oxidation (EOM). Meanwhile, the pH-asymmetric integrated cell with simulated and complex waste acid-base solutions showcases a lower voltage of 1.62 V at 1 mA applied current with the same catalysts. Additionally, the device demonstrates negligible degradation at 10 mA cm−2, suggesting high overall durability for EOM. Techno-economic evaluation is also conducted to present the energy-saving and profit advantages with less oxygen evolution reaction (OER) competition. This work opens a pathway for prospective applications for the production of commodity chemicals simultaneously in one electrolyzer, which can even turn waste acid and base into resources.”
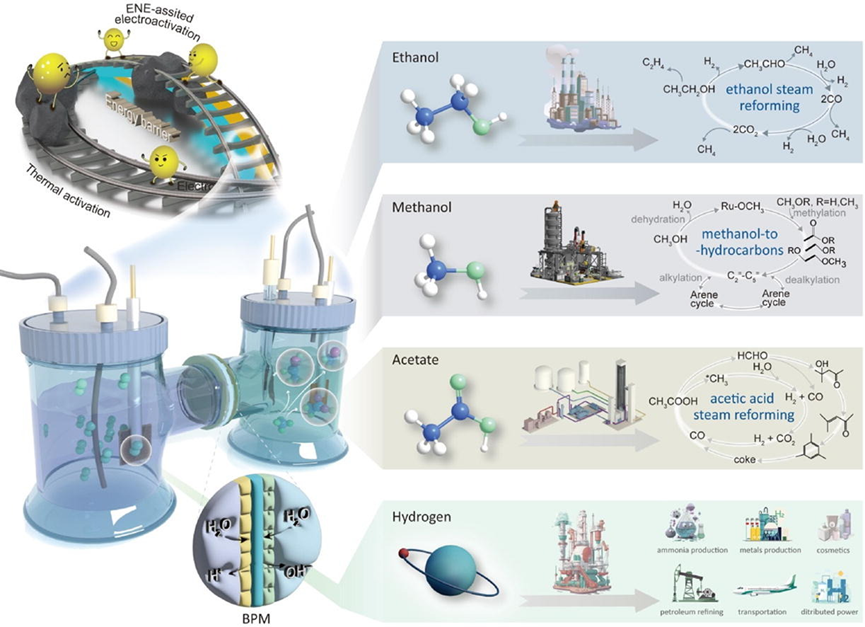
Figure 1. The graphical abstract of the innovative electrolyzer reported in this work.
This work is supported by the Shanghai Key Laboratory of Hydrogen Science & Center of Hydrogen Science of Shanghai Jiao Tong University, and Shanghai High-Level Oversea Talents Award, etc.
Read the full article: https://doi.org/10.1016/j.scib.2025.05.031
