A joint research team led by Professor Hongjing Dou and Professor Stephen Mann from the School of Materials Science and Engineering at Shanghai Jiao Tong University has recently published a research paper titled "Mechano-crosstalk between living and artificial cells" in Nature Communications. This work systematically reveals the mechanotransduction mechanism and polarization regulation of macrophages during direct cell contact by constructing a series of artificial cells mimicking pathogens with tunable mechanical properties. Xiaolei Yu (Class of 2020 direct-entry PhD student, 2025 graduate, now a postdoctoral fellow at Shanghai Jiao Tong University) is the sole first author of the paper. Co-authors include Dr. Vincent Mukwaya from the School of Materials Science and Engineering at Shanghai Jiao Tong University, Director Li Wang from Ruijin Hospital Affiliated with Shanghai Jiao Tong University School of Medicine, and Professor Weili Zhao. The corresponding authors are Professor Hongjing Dou and Professor Stephen Mann from the School of Materials Science and Engineering at Shanghai Jiao Tong University.
Link to the paper: https://doi.org/10.1038/s41467-025-63581-1
Cells perceiving and decoding the mechanical properties of adjacent cells through direct contact is a fundamental pathway for regulating their own behavior. As core immune cells, macrophages are highly migratory and capable of sensitively detecting changes in mechanical signals within the microenvironment, thereby regulating their polarization state and immune function to maintain tissue homeostasis. However, bottlenecks in existing models and tools have hindered the elucidation of the deep mechanisms underlying direct mechanical interactions between cells.
The research team first established a novel all-aqueous phase system for constructing cell-like materials, enabling the batch production of compartmentalized, cell-like materials with homogeneous properties and flexibly tunable parameters. Through functional modifications of their interior and surface, they successfully constructed artificial cells mimicking pathogens with a series of stiffness gradients within the physiologically relevant stiffness range (Figure 1).
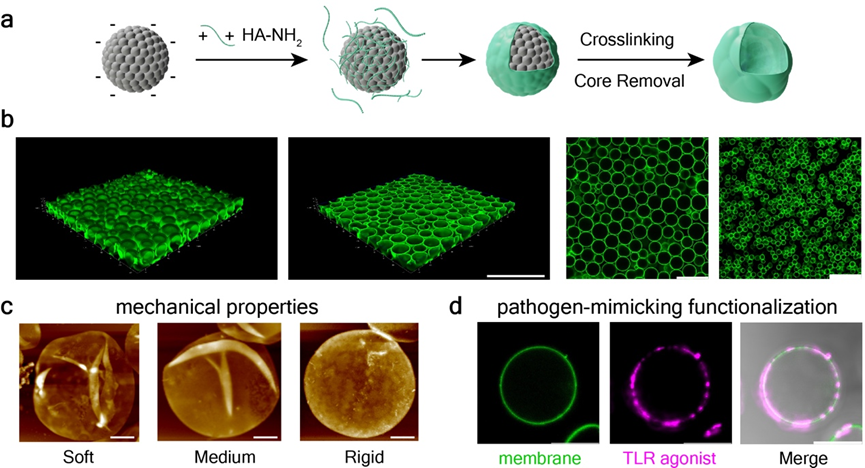
Figure 1. Construction, functional modification, and mechanical property tuning of artificial pathogen-like cells.
In vitro co-culture experiments showed that increasing the stiffness of the artificial pathogen-like cells significantly promoted the polarization of macrophages towards a pro-inflammatory phenotype. Microscopic observation revealed the formation of numerous macrophage pseudopods at the interaction interface. This phenomenon was verified across artificial pathogen-like cells of different sizes, surface chemical modifications, and in primary macrophages, confirming the universality of pseudopod-mediated polarization responses in macrophages (Figure 2).
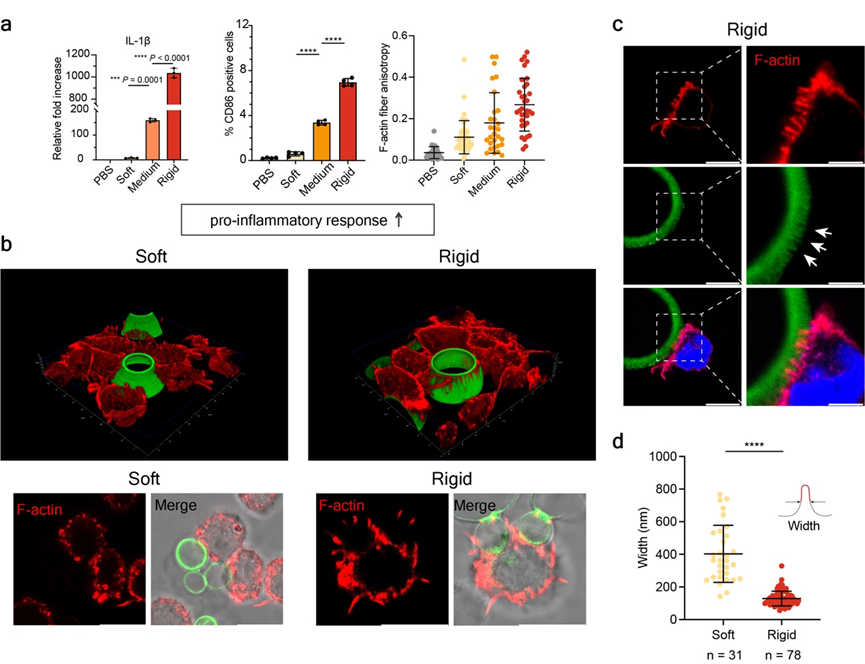
Figure 2. Rigid artificial cell interfaces promote macrophage sensing and polarization response via pseudopods.
The research team further focused on the artificial cell–macrophage interaction interface. Using techniques such as immunofluorescence, inhibitor treatment, and specific gene knockout, they confirmed the existence of a "molecular clutch" structure at the interaction interface, formed by the connection between the glycocalyx component hyaluronic acid on the artificial cell membrane, the CD44 receptor on the macrophage surface, and the cytoskeletal F-actin. Mechanical measurements using DNA-based tension probes further demonstrated that increased stiffness of the artificial cells promotes the assembly of this clutch and the transmission of intracellular forces (Figure 3).
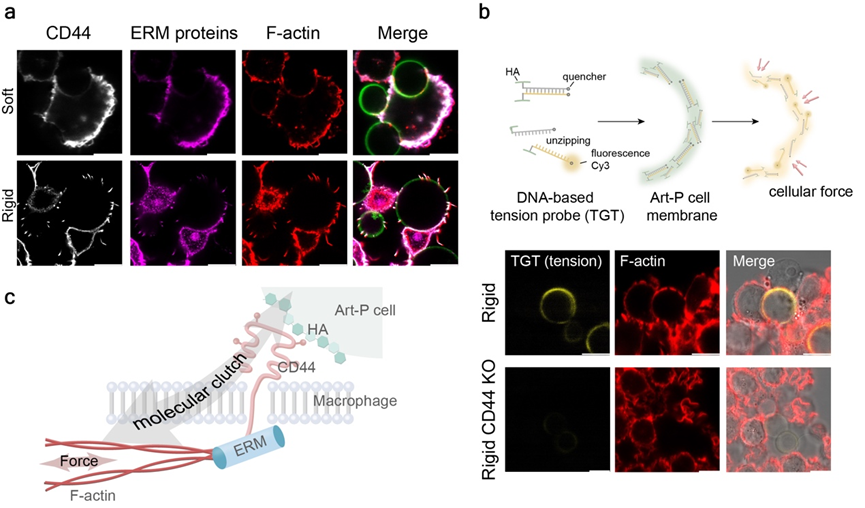
Figure 3. Molecular clutch engagement and force transmission at the interaction interface promote mechanotransduction.
As cell polarization is ultimately regulated at the level of gene expression, a biochemical process, the team further investigated the mechano-biochemical signal transduction occurring within macrophages. The results indicated that the macrophage membrane mechanosensitive ion channel Piezo1 and the RhoA/ROCK signaling pathway play key roles in this mechano-biochemical signal transduction, jointly regulating the expression of pro-inflammatory-related genes (Figure 4).
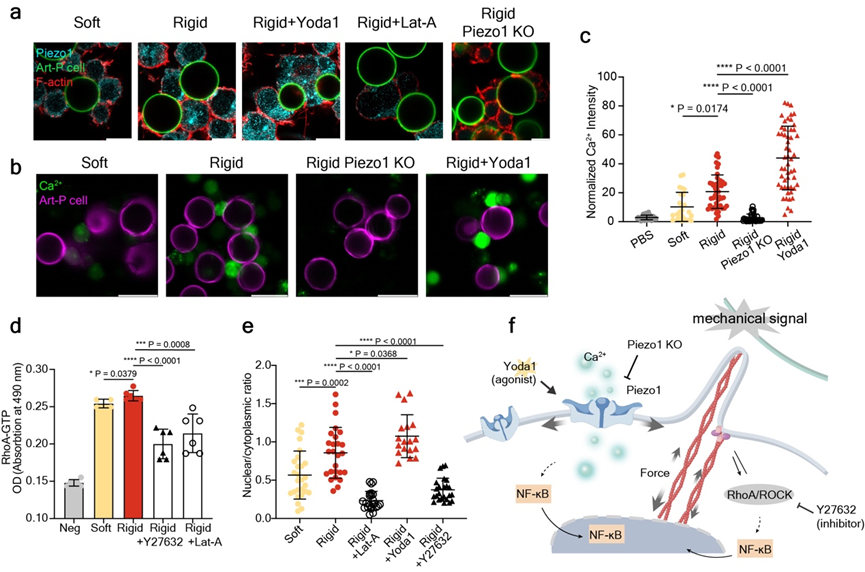
Figure 4. Mechano-biochemical signal transduction in macrophages.
This research demonstrates the potential of biomimetic artificial cells in investigating intercellular interaction mechanisms, providing a new tool for understanding complex signal perception and plastic polarization in immune cells. Based on these findings, the research team is committed to advancing and optimizing the biomimetic construction of this artificial cell system and exploring its potential for clinical translation as a new strategy for immunotherapy.
